Both human ferredoxins 1 and 2 and ferredoxin reductase are important for iron-sulfur cluster biogenesis
- PMID: 22101253
- PMCID: PMC3546607
- DOI: 10.1016/j.bbamcr.2011.11.002
Both human ferredoxins 1 and 2 and ferredoxin reductase are important for iron-sulfur cluster biogenesis
Abstract
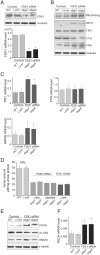
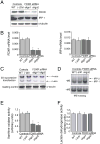
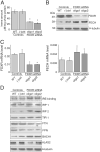
Ferredoxins are iron-sulfur proteins that have been studied for decades because of their role in facilitating the monooxygenase reactions catalyzed by p450 enzymes. More recently, studies in bacteria and yeast have demonstrated important roles for ferredoxin and ferredoxin reductase in iron-sulfur cluster assembly. The human genome contains two homologous ferredoxins, ferredoxin 1 (FDX1) and ferredoxin 2 (FDX2--formerly known as ferredoxin 1L). More recently, the roles of these two human ferredoxins in iron-sulfur cluster assembly were assessed, and it was concluded that FDX1 was important solely for its interaction with p450 enzymes to synthesize mitochondrial steroid precursors, whereas FDX2 was used for synthesis of iron-sulfur clusters, but not steroidogenesis. To further assess the role of the FDX-FDXR system in mammalian iron-sulfur cluster biogenesis, we performed siRNA studies on FDX1 and FDX2, on several human cell lines, using oligonucleotides identical to those previously used, along with new oligonucleotides that specifically targeted each gene. We concluded that both FDX1 and FDX2 were important in iron-sulfur cluster biogenesis. Loss of FDX1 activity disrupted activity of iron-sulfur cluster enzymes and cellular iron homeostasis, causing mitochondrial iron overload and cytosolic iron depletion. Moreover, knockdown of the sole human ferredoxin reductase, FDXR, diminished iron-sulfur cluster assembly and caused mitochondrial iron overload in conjunction with cytosolic depletion. Our studies suggest that interference with any of the three related genes, FDX1, FDX2 or FDXR, disrupts iron-sulfur cluster assembly and maintenance of normal cytosolic and mitochondrial iron homeostasis.
Published by Elsevier B.V.
Conflict of interest statement
Figures





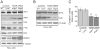
Similar articles
-
Human Mitochondrial Ferredoxin 1 (FDX1) and Ferredoxin 2 (FDX2) Both Bind Cysteine Desulfurase and Donate Electrons for Iron-Sulfur Cluster Biosynthesis.Biochemistry. 2017 Jan 24;56(3):487-499. doi: 10.1021/acs.biochem.6b00447. Epub 2017 Jan 11. Biochemistry. 2017. PMID: 28001042 Free PMC article.
-
Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis.Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11775-80. doi: 10.1073/pnas.1004250107. Epub 2010 Jun 14. Proc Natl Acad Sci U S A. 2010. PMID: 20547883 Free PMC article.
-
Functional spectrum and specificity of mitochondrial ferredoxins FDX1 and FDX2.Nat Chem Biol. 2023 Feb;19(2):206-217. doi: 10.1038/s41589-022-01159-4. Epub 2022 Oct 24. Nat Chem Biol. 2023. PMID: 36280795 Free PMC article.
-
Mitochondrial [2Fe-2S] ferredoxins: new functions for old dogs.FEBS Lett. 2023 Jan;597(1):102-121. doi: 10.1002/1873-3468.14546. Epub 2022 Dec 7. FEBS Lett. 2023. PMID: 36443530 Review.
-
Mechanisms of Mitochondrial Iron-Sulfur Protein Biogenesis.Annu Rev Biochem. 2020 Jun 20;89:471-499. doi: 10.1146/annurev-biochem-013118-111540. Epub 2020 Jan 14. Annu Rev Biochem. 2020. PMID: 31935115 Review.
Cited by
-
Development and validation of a novel diagnostic model for musculoskeletal aging (sarcopenia) based on cuproptosis-related genes associated with immunity.Am J Transl Res. 2022 Dec 15;14(12):8523-8538. eCollection 2022. Am J Transl Res. 2022. PMID: 36628249 Free PMC article.
-
p53 tumor suppressor and iron homeostasis.FEBS J. 2019 Feb;286(4):620-629. doi: 10.1111/febs.14638. Epub 2018 Sep 4. FEBS J. 2019. PMID: 30133149 Free PMC article. Review.
-
Mammalian iron sulfur cluster biogenesis: From assembly to delivery to recipient proteins with a focus on novel targets of the chaperone and co-chaperone proteins.IUBMB Life. 2022 Jul;74(7):684-704. doi: 10.1002/iub.2593. Epub 2022 Jan 25. IUBMB Life. 2022. PMID: 35080107 Free PMC article. No abstract available.
-
Revealing the key role of cuproptosis in osteoporosis via the bioinformatic analysis and experimental validation of cuproptosis-related genes.Mamm Genome. 2024 Sep;35(3):414-431. doi: 10.1007/s00335-024-10049-0. Epub 2024 Jun 21. Mamm Genome. 2024. PMID: 38904833
-
Structure of human Fe-S assembly subcomplex reveals unexpected cysteine desulfurase architecture and acyl-ACP-ISD11 interactions.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):E5325-E5334. doi: 10.1073/pnas.1702849114. Epub 2017 Jun 20. Proc Natl Acad Sci U S A. 2017. PMID: 28634302 Free PMC article.
References
-
- Ewen KM, Kleser M, Bernhardt R. Adrenodoxin: the archetype of vertebratetype [2Fe–2S] cluster ferredoxins. Biochim Biophys Acta. 2011;1814:111–125. - PubMed
-
- Vickery LE. Molecular recognition and electron transfer in mitochondrial steroid hydroxylase systems. Steroids. 1997;62:124–127. - PubMed
-
- Zheng L, Cash VL, Flint DH, Dean DR. Assembly of iron–sulfur clusters. Identification of an iscSUA-hscBA-FDX gene cluster from Azotobacter vinelandii. J Biol Chem. 1998;273:13264–13272. - PubMed
-
- Jung YS, Gao-Sheridan HS, Christiansen J, Dean DR, Burgess BK. Purification and biophysical characterization of a new [2Fe–2S] ferredoxin from Azotobacter vinelandii, a putative [Fe–S] cluster assembly/repair protein. J Biol Chem. 1999;274:32402–32410. - PubMed
-
- Tokumoto U, Takahashi Y. Genetic analysis of the isc operon in Escherichia coli involved in the biogenesis of cellular iron–sulfur proteins. J Biochem. 2001;130:63–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

