GM-CSF-induced regulatory T cells selectively inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis
- PMID: 22099723
- PMCID: PMC3234297
- DOI: 10.1016/j.jneuroim.2011.10.010
GM-CSF-induced regulatory T cells selectively inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis
Abstract
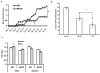
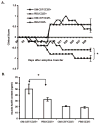
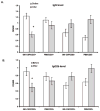
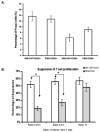
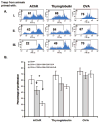
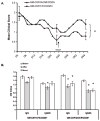
We and others have demonstrated the ability of granulocyte-macrophage colony-stimulating factor (GM-CSF) to suppress autoimmunity by increasing the number of CD4(+)CD25(+) regulatory T cells (Tregs). In the current study, we have explored the critical role of induced antigen specific Tregs in the therapeutic effects of GM-CSF in murine experimental autoimmune myasthenia gravis (EAMG). Specifically, we show that Tregs from GM-CSF treated EAMG mice (GM-CSF/AChR-induced-Tregs) adoptively transferred into animals with EAMG suppressed clinical disease more potently than equal numbers of Tregs from either GM-CSF untreated EAMG mice or healthy mice treated with GM-CSF. In addition, GM-CSF/AChR-induced-Tregs selectively suppressed antigen specific T cell proliferation induced by AChR relative to that induced by an irrelevant self antigen, (thyroglobulin) and failed to significantly alter T cell proliferation in response to an exogenous antigen (ovalbumin). These results are consistent with the hypothesized mechanism of action of GM-CSF involving the mobilization of tolerogenic dendritic cell precursors which, upon antigen (AChR) capture, suppress the anti-AChR immune response through the induction/expansion of AChR-specific Tregs.
Copyright © 2011. Published by Elsevier B.V.
Figures

Similar articles
-
Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia gravis.Clin Immunol. 2008 Aug;128(2):172-80. doi: 10.1016/j.clim.2008.03.509. Epub 2008 May 27. Clin Immunol. 2008. PMID: 18502693 Free PMC article.
-
CD1d(hi)CD5+ B cells expanded by GM-CSF in vivo suppress experimental autoimmune myasthenia gravis.J Immunol. 2014 Sep 15;193(6):2669-77. doi: 10.4049/jimmunol.1303397. Epub 2014 Aug 18. J Immunol. 2014. PMID: 25135828 Free PMC article.
-
Strategies for treating autoimmunity: novel insights from experimental myasthenia gravis.Ann N Y Acad Sci. 2008;1132:276-82. doi: 10.1196/annals.1405.023. Ann N Y Acad Sci. 2008. PMID: 18567878 Free PMC article.
-
Novel animal models of acetylcholine receptor antibody-related myasthenia gravis.Ann N Y Acad Sci. 2012 Dec;1274:133-9. doi: 10.1111/j.1749-6632.2012.06773.x. Ann N Y Acad Sci. 2012. PMID: 23252908 Review.
-
Animal models of myasthenia gravis.Clin Immunol. 2000 Feb;94(2):75-87. doi: 10.1006/clim.1999.4807. Clin Immunol. 2000. PMID: 10637092 Review.
Cited by
-
Microglial phenotypes in Parkinson's disease and animal models of the disease.Prog Neurobiol. 2017 Aug;155:57-75. doi: 10.1016/j.pneurobio.2016.04.006. Epub 2016 Apr 20. Prog Neurobiol. 2017. PMID: 27107797 Free PMC article. Review.
-
Advances in autoimmune myasthenia gravis management.Expert Rev Neurother. 2018 Jul;18(7):573-588. doi: 10.1080/14737175.2018.1491310. Epub 2018 Jul 4. Expert Rev Neurother. 2018. PMID: 29932785 Free PMC article. Review.
-
GM-CSF-neuroantigen fusion proteins reverse experimental autoimmune encephalomyelitis and mediate tolerogenic activity in adjuvant-primed environments: association with inflammation-dependent, inhibitory antigen presentation.J Immunol. 2014 Sep 1;193(5):2317-29. doi: 10.4049/jimmunol.1303223. Epub 2014 Jul 21. J Immunol. 2014. PMID: 25049359 Free PMC article.
-
Bridge between neuroimmunity and traumatic brain injury.Curr Pharm Des. 2014;20(26):4284-98. Curr Pharm Des. 2014. PMID: 24025052 Free PMC article. Review.
-
The Application Potential of the Regulation of Tregs Function by Irisin in the Prevention and Treatment of Immune-Related Diseases.Drug Des Devel Ther. 2024 Jul 16;18:3005-3023. doi: 10.2147/DDDT.S465713. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39050796 Free PMC article. Review.
References
-
- Askenasy N, Kaminitz A, Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun Rev. 2008;7:370–375. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nature Rev. 2003;3:253–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

