Genetic mapping of a highly variable norovirus GII.4 blockade epitope: potential role in escape from human herd immunity
- PMID: 22090110
- PMCID: PMC3255819
- DOI: 10.1128/JVI.06189-11
Genetic mapping of a highly variable norovirus GII.4 blockade epitope: potential role in escape from human herd immunity
Abstract
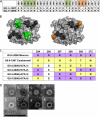
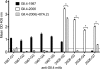
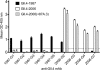
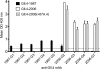
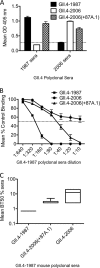
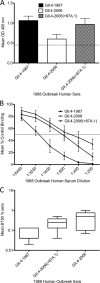
Noroviruses account for 96% of viral gastroenteritis cases worldwide, with GII.4 strains responsible >80% of norovirus outbreaks. Histo-blood group antigens (HBGAs) are norovirus binding ligands, and antigenic and preferential HBGA binding profiles vary over time as new GII.4 strains emerge. The capsid P2 subdomain facilitates HBGA binding, contains neutralizing antibody epitopes, and likely evolves in response to herd immunity. To identify amino acids regulating HBGA binding and antigenic differences over time, we created chimeric virus-like particles (VLPs) between the GII.4-1987 and GII.4-2006 strains by exchanging amino acids in putative epitopes and characterized their antigenic and HBGA binding profiles using anti-GII.4-1987 and -2006 mouse monoclonal antibodies (MAbs) and polyclonal sera, 1988 outbreak human sera, and synthetic HBGAs. The exchange of amino acids 393 to 395 between GII.4-1987 and GII.4-2006 resulted in altered synthetic HBGA binding compared to parental strains. Introduction of GII.4-1987 residues 294, 297 to 298, 368, and 372 (epitope A) into GII.4-2006 resulted in reactivity with three anti-GII.4-1987 MAbs and reduced reactivity with four anti-GII.4-2006 MAbs. The three anti-GII.4-1987 MAbs also blocked chimeric VLP-HBGA interaction, while an anti-GII.4-2006 blocking antibody did not, indicating that epitope A amino acids comprise a potential neutralizing epitope for GII.4-1987 and GII.4-2006. We also tested GII.4-1987-immunized mouse polyclonal sera and 1988 outbreak human sera for the ability to block chimeric VLP-HBGA interaction and found that epitope A amino acids contribute significantly to the GII.4-1987 blockade response. Our data provide insights that help explain the emergence of new GII.4 epidemic strains over time, may aid development of norovirus therapeutics, and may help predict the emergence of future epidemic strains.
Figures
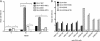
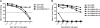
Similar articles
-
Human Norovirus Neutralized by a Monoclonal Antibody Targeting the Histo-Blood Group Antigen Pocket.J Virol. 2019 Feb 19;93(5):e02174-18. doi: 10.1128/JVI.02174-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541855 Free PMC article.
-
Immunogenetic mechanisms driving norovirus GII.4 antigenic variation.PLoS Pathog. 2012;8(5):e1002705. doi: 10.1371/journal.ppat.1002705. Epub 2012 May 17. PLoS Pathog. 2012. PMID: 22615565 Free PMC article.
-
Identification and characterization of antibody-binding epitopes on the norovirus GII.3 capsid.J Virol. 2014 Feb;88(4):1942-52. doi: 10.1128/JVI.02992-13. Epub 2013 Nov 27. J Virol. 2014. PMID: 24284328 Free PMC article.
-
The Antigenic Topology of Norovirus as Defined by B and T Cell Epitope Mapping: Implications for Universal Vaccines and Therapeutics.Viruses. 2019 May 10;11(5):432. doi: 10.3390/v11050432. Viruses. 2019. PMID: 31083353 Free PMC article. Review.
-
Norovirus pathogenesis: mechanisms of persistence and immune evasion in human populations.Immunol Rev. 2008 Oct;225:190-211. doi: 10.1111/j.1600-065X.2008.00680.x. Immunol Rev. 2008. PMID: 18837783 Review.
Cited by
-
Emerging Novel GII.P16 Noroviruses Associated with Multiple Capsid Genotypes.Viruses. 2019 Jun 8;11(6):535. doi: 10.3390/v11060535. Viruses. 2019. PMID: 31181749 Free PMC article.
-
Prolonged norovirus infections correlate to quasispecies evolution resulting in structural changes of surface-exposed epitopes.iScience. 2021 Jun 30;24(7):102802. doi: 10.1016/j.isci.2021.102802. eCollection 2021 Jul 23. iScience. 2021. PMID: 34355146 Free PMC article.
-
Evolutionary Constraints on the Norovirus Pandemic Variant GII.4_2006b over the Five-Year Persistence in Japan.Front Microbiol. 2017 Mar 13;8:410. doi: 10.3389/fmicb.2017.00410. eCollection 2017. Front Microbiol. 2017. PMID: 28348551 Free PMC article.
-
Identification of a blockade epitope of human norovirus GII.17.Emerg Microbes Infect. 2021 Dec;10(1):954-963. doi: 10.1080/22221751.2021.1925162. Emerg Microbes Infect. 2021. PMID: 33929932 Free PMC article.
-
Genomic analysis of human noroviruses using combined Illumina-Nanopore data.Virus Evol. 2021 Sep 15;7(2):veab079. doi: 10.1093/ve/veab079. eCollection 2021. Virus Evol. 2021. PMID: 35186325 Free PMC article.
References
-
- Atmar RL, Estes MK. 2006. The epidemiologic and clinical importance of norovirus infection. Gastroenterol. Clin. North Am. 35:275–290, viii - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

