Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor κB-mediated pathogenic pathways
- PMID: 22084410
- PMCID: PMC3256969
- DOI: 10.1084/jem.20111313
Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor κB-mediated pathogenic pathways
Abstract
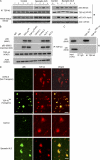
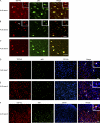
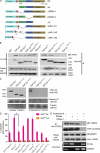
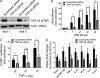
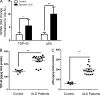
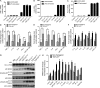
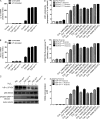
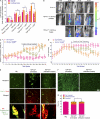
TDP-43 (TAR DNA-binding protein 43) inclusions are a hallmark of amyotrophic lateral sclerosis (ALS). In this study, we report that TDP-43 and nuclear factor κB (NF-κB) p65 messenger RNA and protein expression is higher in spinal cords in ALS patients than healthy individuals. TDP-43 interacts with and colocalizes with p65 in glial and neuronal cells from ALS patients and mice expressing wild-type and mutant TDP-43 transgenes but not in cells from healthy individuals or nontransgenic mice. TDP-43 acted as a co-activator of p65, and glial cells expressing higher amounts of TDP-43 produced more proinflammatory cytokines and neurotoxic mediators after stimulation with lipopolysaccharide or reactive oxygen species. TDP-43 overexpression in neurons also increased their vulnerability to toxic mediators. Treatment of TDP-43 mice with Withaferin A, an inhibitor of NF-κB activity, reduced denervation in the neuromuscular junction and ALS disease symptoms. We propose that TDP-43 deregulation contributes to ALS pathogenesis in part by enhancing NF-κB activation and that NF-κB may constitute a therapeutic target for the disease.
Figures

Similar articles
-
Mitigation of ALS Pathology by Neuron-Specific Inhibition of Nuclear Factor Kappa B Signaling.J Neurosci. 2020 Jun 24;40(26):5137-5154. doi: 10.1523/JNEUROSCI.0536-20.2020. Epub 2020 May 26. J Neurosci. 2020. PMID: 32457070 Free PMC article.
-
Withania somnifera Reverses Transactive Response DNA Binding Protein 43 Proteinopathy in a Mouse Model of Amyotrophic Lateral Sclerosis/Frontotemporal Lobar Degeneration.Neurotherapeutics. 2017 Apr;14(2):447-462. doi: 10.1007/s13311-016-0499-2. Neurotherapeutics. 2017. PMID: 27928708 Free PMC article.
-
Ubiquilin-2 drives NF-κB activity and cytosolic TDP-43 aggregation in neuronal cells.Mol Brain. 2015 Oct 31;8(1):71. doi: 10.1186/s13041-015-0162-6. Mol Brain. 2015. PMID: 26521126 Free PMC article.
-
The molecular link between inefficient GluA2 Q/R site-RNA editing and TDP-43 pathology in motor neurons of sporadic amyotrophic lateral sclerosis patients.Brain Res. 2014 Oct 10;1584:28-38. doi: 10.1016/j.brainres.2013.12.011. Epub 2013 Dec 16. Brain Res. 2014. PMID: 24355598 Review.
-
Review: Prion-like mechanisms of transactive response DNA binding protein of 43 kDa (TDP-43) in amyotrophic lateral sclerosis (ALS).Neuropathol Appl Neurobiol. 2015 Aug;41(5):578-97. doi: 10.1111/nan.12206. Epub 2015 Apr 20. Neuropathol Appl Neurobiol. 2015. PMID: 25487060 Review.
Cited by
-
Identification of quantitative trait loci for survival in the mutant dynactin p150Glued mouse model of motor neuron disease.PLoS One. 2022 Sep 15;17(9):e0274615. doi: 10.1371/journal.pone.0274615. eCollection 2022. PLoS One. 2022. PMID: 36107978 Free PMC article.
-
Mitochondria Dysfunction and Neuroinflammation in Neurodegeneration: Who Comes First?Antioxidants (Basel). 2024 Feb 16;13(2):240. doi: 10.3390/antiox13020240. Antioxidants (Basel). 2024. PMID: 38397838 Free PMC article. Review.
-
The changing scene of amyotrophic lateral sclerosis.Nat Rev Neurosci. 2013 Apr;14(4):248-64. doi: 10.1038/nrn3430. Epub 2013 Mar 6. Nat Rev Neurosci. 2013. PMID: 23463272 Review.
-
From Transcriptome to Noncoding RNAs: Implications in ALS Mechanism.Neurol Res Int. 2012;2012:278725. doi: 10.1155/2012/278725. Epub 2012 Jun 17. Neurol Res Int. 2012. PMID: 22778949 Free PMC article.
-
Axonal transport deficits and neurodegenerative diseases.Nat Rev Neurosci. 2013 Mar;14(3):161-76. doi: 10.1038/nrn3380. Epub 2013 Jan 30. Nat Rev Neurosci. 2013. PMID: 23361386 Review.
References
-
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., Oda T. 2006. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351:602–611 10.1016/j.bbrc.2006.10.093 - DOI - PubMed
-
- Badadani M., Nalbandian A., Watts G.D., Vesa J., Kitazawa M., Su H., Tanaja J., Dec E., Wallace D.C., Mukherjee J., et al. 2010. VCP associated inclusion body myopathy and paget disease of bone knock-in mouse model exhibits tissue pathology typical of human disease. PLoS ONE. 5:e13183 10.1371/journal.pone.0013183 - DOI - PMC - PubMed
-
- Bergmann M., Hart L., Lindsay M., Barnes P.J., Newton R. 1998. IkappaBalpha degradation and nuclear factor-kappaB DNA binding are insufficient for interleukin-1beta and tumor necrosis factor-alpha-induced kappaB-dependent transcription. Requirement for an additional activation pathway. J. Biol. Chem. 273:6607–6610 10.1074/jbc.273.12.6607 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

