In vivo fate mapping identifies mesenchymal progenitor cells
- PMID: 22083974
- PMCID: PMC3560295
- DOI: 10.1002/stem.780
In vivo fate mapping identifies mesenchymal progenitor cells
Abstract
Adult mesenchymal progenitor cells have enormous potential for use in regenerative medicine. However, the true identity of the progenitors in vivo and their progeny has not been precisely defined. We hypothesize that cells expressing a smooth muscle α-actin promoter (αSMA)-directed Cre transgene represent mesenchymal progenitors of adult bone tissue. By combining complementary colors in combination with transgenes activating at mature stages of the lineage, we characterized the phenotype and confirmed the ability of isolated αSMA(+) cells to progress from a progenitor to fully mature state. In vivo lineage tracing experiments using a new bone formation model confirmed the osteogenic phenotype of αSMA(+) cells. In vitro analysis of the in vivo-labeled SMA9(+) cells supported their differentiation potential into mesenchymal lineages. Using a fracture-healing model, αSMA9(+) cells served as a pool of fibrocartilage and skeletal progenitors. Confirmation of the transition of αSMA9(+) progenitor cells to mature osteoblasts during fracture healing was assessed by activation of bone-specific Col2.3emd transgene. Our findings provide a novel in vivo identification of defined population of mesenchymal progenitor cells with active role in bone remodeling and regeneration.
Copyright © 2011 AlphaMed Press.
Figures

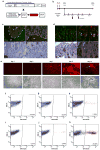
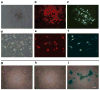
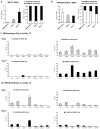


Similar articles
-
In vivo identification of periodontal progenitor cells.J Dent Res. 2013 Aug;92(8):709-15. doi: 10.1177/0022034513493434. Epub 2013 Jun 4. J Dent Res. 2013. PMID: 23735585 Free PMC article.
-
Analysis of αSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing.J Bone Miner Res. 2014;29(5):1283-94. doi: 10.1002/jbmr.2140. J Bone Miner Res. 2014. PMID: 24190076 Free PMC article.
-
Lineage tracing of resident tendon progenitor cells during growth and natural healing.PLoS One. 2014 Apr 23;9(4):e96113. doi: 10.1371/journal.pone.0096113. eCollection 2014. PLoS One. 2014. PMID: 24759953 Free PMC article.
-
Circulating endothelial/skeletal progenitor cells for bone regeneration and healing.Bone. 2008 Sep;43(3):434-9. doi: 10.1016/j.bone.2008.05.001. Epub 2008 May 10. Bone. 2008. PMID: 18547890 Review.
-
Bone regeneration: the stem/progenitor cells point of view.J Cell Mol Med. 2010 Jan;14(1-2):103-15. doi: 10.1111/j.1582-4934.2009.00878.x. Epub 2009 Aug 10. J Cell Mol Med. 2010. PMID: 19840188 Free PMC article. Review.
Cited by
-
Real-Time Imaging of CCL5-Induced Migration of Periosteal Skeletal Stem Cells in Mice.J Vis Exp. 2020 Sep 16;(163):10.3791/61162. doi: 10.3791/61162. J Vis Exp. 2020. PMID: 33016934 Free PMC article.
-
Versatile subtypes of pericytes and their roles in spinal cord injury repair, bone development and repair.Bone Res. 2022 Mar 16;10(1):30. doi: 10.1038/s41413-022-00203-2. Bone Res. 2022. PMID: 35296645 Free PMC article. Review.
-
Lineage Tracing of RGS5-CreER-Labeled Cells in Long Bones During Homeostasis and Injury.Stem Cells. 2023 May 15;41(5):493-504. doi: 10.1093/stmcls/sxad020. Stem Cells. 2023. PMID: 36888549 Free PMC article.
-
Skeletal Stem/Progenitor Cells in Periosteum and Skeletal Muscle Share a Common Molecular Response to Bone Injury.J Bone Miner Res. 2022 Aug;37(8):1545-1561. doi: 10.1002/jbmr.4616. Epub 2022 Jun 29. J Bone Miner Res. 2022. PMID: 35652423 Free PMC article.
-
Cebpb Regulates Skeletal Stem Cell Osteogenic Differentiation and Fracture Healing via the WNT/β-Catenin Pathway.Stem Cells Int. 2022 Jul 18;2022:2091615. doi: 10.1155/2022/2091615. eCollection 2022. Stem Cells Int. 2022. PMID: 35898655 Free PMC article.
References
-
- Bianco P, Riminucci M, Gronthos S, et al. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. - PubMed
-
- Gronthos S, Zannettino AC, Hay SJ, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. - PubMed
-
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. - PubMed
-
- Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. - PubMed
-
- Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

