Generating human intestinal tissue from pluripotent stem cells in vitro
- PMID: 22082986
- PMCID: PMC3896236
- DOI: 10.1038/nprot.2011.410
Generating human intestinal tissue from pluripotent stem cells in vitro
Abstract
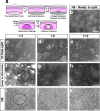
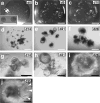
Here we describe a protocol for generating 3D human intestinal tissues (called organoids) in vitro from human pluripotent stem cells (hPSCs). To generate intestinal organoids, pluripotent stem cells are first differentiated into FOXA2(+)SOX17(+) endoderm by treating the cells with activin A for 3 d. After endoderm induction, the pluripotent stem cells are patterned into CDX2(+) mid- and hindgut tissue using FGF4 and WNT3a. During this patterning step, 3D mid- or hindgut spheroids bud from the monolayer epithelium attached to the tissue culture dish. The 3D spheroids are further cultured in Matrigel along with prointestinal growth factors, and they proliferate and expand over 1-3 months to give rise to intestinal tissue, complete with intestinal mesenchyme and epithelium comprising all of the major intestinal cell types. To date, this is the only method for efficiently directing the differentiation of hPSCs into 3D human intestinal tissue in vitro.
Conflict of interest statement
J.M.W. and J.R.S. are inventors on a patent involving the system described herein.
Figures

Similar articles
-
Intestinal Commitment and Maturation of Human Pluripotent Stem Cells Is Independent of Exogenous FGF4 and R-spondin1.PLoS One. 2015 Jul 31;10(7):e0134551. doi: 10.1371/journal.pone.0134551. eCollection 2015. PLoS One. 2015. PMID: 26230325 Free PMC article.
-
Generation of Gastrointestinal Organoids from Human Pluripotent Stem Cells.Methods Mol Biol. 2017;1597:167-177. doi: 10.1007/978-1-4939-6949-4_12. Methods Mol Biol. 2017. PMID: 28361317
-
Generation of small intestinal organoids for experimental intestinal physiology.Methods Cell Biol. 2020;159:143-174. doi: 10.1016/bs.mcb.2020.03.007. Epub 2020 May 4. Methods Cell Biol. 2020. PMID: 32586441
-
Generating human intestinal tissues from pluripotent stem cells to study development and disease.EMBO J. 2015 May 5;34(9):1149-63. doi: 10.15252/embj.201490686. Epub 2015 Mar 19. EMBO J. 2015. PMID: 25792515 Free PMC article. Review.
-
[From human pluripotent stem cells to custom-made intestinal organoids].Med Sci (Paris). 2019 Jun-Jul;35(6-7):549-555. doi: 10.1051/medsci/2019096. Epub 2019 Jul 5. Med Sci (Paris). 2019. PMID: 31274085 Review. French.
Cited by
-
Measuring the elastic modulus of soft culture surfaces and three-dimensional hydrogels using atomic force microscopy.Nat Protoc. 2021 May;16(5):2418-2449. doi: 10.1038/s41596-021-00495-4. Epub 2021 Apr 14. Nat Protoc. 2021. PMID: 33854255 Free PMC article.
-
Probiotic Properties of Escherichia coli Nissle in Human Intestinal Organoids.mBio. 2020 Jul 7;11(4):e01470-20. doi: 10.1128/mBio.01470-20. mBio. 2020. PMID: 32636253 Free PMC article.
-
hPSC-derived organoids: models of human development and disease.J Mol Med (Berl). 2021 Apr;99(4):463-473. doi: 10.1007/s00109-020-01969-w. Epub 2020 Aug 28. J Mol Med (Berl). 2021. PMID: 32857169 Free PMC article. Review.
-
Intestinal Commitment and Maturation of Human Pluripotent Stem Cells Is Independent of Exogenous FGF4 and R-spondin1.PLoS One. 2015 Jul 31;10(7):e0134551. doi: 10.1371/journal.pone.0134551. eCollection 2015. PLoS One. 2015. PMID: 26230325 Free PMC article.
-
Intestinal organoids: roadmap to the clinic.Am J Physiol Gastrointest Liver Physiol. 2021 Jul 1;321(1):G1-G10. doi: 10.1152/ajpgi.00425.2020. Epub 2021 May 5. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33950707 Free PMC article. Review.
References
-
- Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

