G-protein βγ subunits in vasorelaxing and anti-endothelinergic effects of calcitonin gene-related peptide
- PMID: 22074193
- PMCID: PMC3415655
- DOI: 10.1111/j.1476-5381.2011.01774.x
G-protein βγ subunits in vasorelaxing and anti-endothelinergic effects of calcitonin gene-related peptide
Abstract
Background and purpose: Calcitonin gene-related peptide (CGRP) has been proposed to relax vascular smooth muscle cells (VSMC) via cAMP and can promote dissociation of endothelin-1 (ET-1) from ET(A) receptors. The latter is not mimicked by other stimuli of adenylate cyclases. Therefore, we evaluated the involvement of G-protein βγ subunits (Gβγ) in the arterial effects of CGRP receptor stimulation.
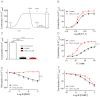
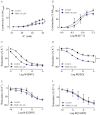
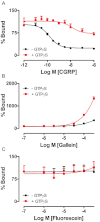
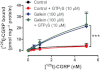
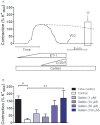
Experimental approach: To test the hypothesis that instead of α subunits of G-proteins (Gαs), Gβγ mediates the effects of CGRP receptor activation, we used (i) rat isolated mesenteric resistance arteries (MRA), (ii) pharmacological modulators of cyclic nucleotides; and (iii) low molecular weight inhibitors of the functions of Gβγ, gallein and M119. To validate these tools with respect to CGRP receptor function, we performed organ bath studies with rat isolated MRA, radioligand binding on membranes from CHO cells expressing human CGRP receptors and cAMP production assays in rat cultured VSMC.
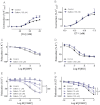
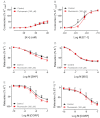
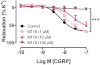
Key results: In isolated arteries contracted with K(+) or ET-1, IBMX (PDE inhibitor) increased sodium nitroprusside (SNP)- and isoprenaline (ISO)- but not CGRP-induced relaxations. While fluorescein (negative control) was without effects, gallein increased binding of [(125) I]-CGRP in the absence and presence of GTPγS. Gallein also increased CGRP-induced cAMP production in VSMC. Despite these stimulating effects, gallein and M119 selectively inhibited the relaxing and anti-endothelinergic effects of CGRP in isolated arteries while not altering contractile responses to K(+) or ET-1 or relaxing responses to ISO or SNP.
Conclusion and implications: Activated CGRP receptors induce cyclic nucleotide-independent relaxation of VSMC and terminate arterial effects of ET-1 via Gβγ.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures

Similar articles
-
Investigating the Role of G Protein βγ in Kv7-Dependent Relaxations of the Rat Vasculature.Arterioscler Thromb Vasc Biol. 2018 Sep;38(9):2091-2102. doi: 10.1161/ATVBAHA.118.311360. Arterioscler Thromb Vasc Biol. 2018. PMID: 30002060 Free PMC article.
-
Intracellular pathways of calcitonin gene-related peptide-induced relaxation of human coronary arteries: A key role for Gβγ subunit instead of cAMP.Br J Pharmacol. 2024 Aug;181(15):2478-2491. doi: 10.1111/bph.16372. Epub 2024 Apr 7. Br J Pharmacol. 2024. PMID: 38583945
-
Binding and functional pharmacological characteristics of gepant-type antagonists in rat brain and mesenteric arteries.Vascul Pharmacol. 2017 Mar;90:36-43. doi: 10.1016/j.vph.2017.02.001. Epub 2017 Feb 10. Vascul Pharmacol. 2017. PMID: 28192258
-
Influence of methanandamide and CGRP on potassium currents in smooth muscle cells of small mesenteric arteries.Pflugers Arch. 2012 Apr;463(5):669-77. doi: 10.1007/s00424-012-1083-1. Epub 2012 Mar 14. Pflugers Arch. 2012. PMID: 22415212
-
Blunted pancreatic polypeptide-induced vasodilatation in mesenteric resistance vessels from spontaneously hypertensive rats.Eur J Pharmacol. 2008 Dec 28;601(1-3):118-23. doi: 10.1016/j.ejphar.2008.09.037. Epub 2008 Sep 30. Eur J Pharmacol. 2008. PMID: 18851959
Cited by
-
Alpha-Calcitonin Gene Related Peptide: New Therapeutic Strategies for the Treatment and Prevention of Cardiovascular Disease and Migraine.Front Physiol. 2022 Feb 11;13:826122. doi: 10.3389/fphys.2022.826122. eCollection 2022. Front Physiol. 2022. PMID: 35222088 Free PMC article. Review.
-
Efficacy of the lumbar sympathetic ganglion block in lower limb pain and its application prospects during the perioperative period.Ibrain. 2022 Oct 9;8(4):442-452. doi: 10.1002/ibra.12069. eCollection 2022 Winter. Ibrain. 2022. PMID: 37786587 Free PMC article. Review.
-
Investigating the Role of G Protein βγ in Kv7-Dependent Relaxations of the Rat Vasculature.Arterioscler Thromb Vasc Biol. 2018 Sep;38(9):2091-2102. doi: 10.1161/ATVBAHA.118.311360. Arterioscler Thromb Vasc Biol. 2018. PMID: 30002060 Free PMC article.
-
Carbamoylated Erythropoietin-Induced Cerebral Blood Perfusion and Vascular Gene Regulation.Int J Mol Sci. 2023 Jul 15;24(14):11507. doi: 10.3390/ijms241411507. Int J Mol Sci. 2023. PMID: 37511274 Free PMC article.
-
Targeting G protein-coupled receptor signalling by blocking G proteins.Nat Rev Drug Discov. 2018 Nov;17(11):789-803. doi: 10.1038/nrd.2018.135. Epub 2018 Sep 28. Nat Rev Drug Discov. 2018. PMID: 30262890 Free PMC article. Review.
References
-
- Bagnato A, Spinella F, Rosano L. The endothelin axis in cancer: the promise and the challenges of molecularly targeted therapy. Can J Physiol Pharmacol. 2008;86:473–484. - PubMed
-
- Bayewitch ML, Avidor-Reiss T, Levy R, Pfeuffer T, Nevo I, Simonds WF, et al. Differential modulation of adenylyl cyclases I and II by various G beta subunits. J Biol Chem. 1998a;273:2273–2276. - PubMed
-
- Bayewitch ML, Avidor-Reiss T, Levy R, Pfeuffer T, Nevo I, Simonds WF, et al. Inhibition of adenylyl cyclase isoforms V and VI by various Gbetagamma subunits. FASEB J. 1998b;12:1019–1025. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

