Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue
- PMID: 22072766
- PMCID: PMC3255839
- DOI: 10.1128/JVI.05842-11
Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue
Abstract
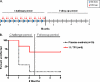
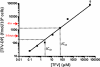
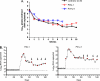
A vaginal gel containing 1% tenofovir (TFV) was found to be safe and effective in reducing HIV infection in women when used pericoitally. Because of the long intracellular half-life of TFV and high drug exposure in vaginal tissues, we hypothesized that a vaginal gel containing TFV may provide long-lasting protection. Here, we performed delayed-challenge experiments and showed that vaginal 1% TFV gel protected 4/6 macaques against vaginal simian-human immunodeficiency virus (SHIV) exposures occurring 3 days after gel application, demonstrating long-lasting protection. Despite continued gel dosing postinfection, neither breakthrough infection had evidence of drug resistance by ultrasensitive testing of SHIV in plasma and vaginal lavage. Analysis of the active intracellular tenofovir diphosphate (TFV-DP) in vaginal lymphocytes collected 4 h to 3 days after gel dosing persistently showed high TFV-DP levels (median, 1,810 fmol/10(6) cells) between 4 and 24 h that exceed the 95% inhibitory concentration (IC(95)), reflecting rapid accumulation and long persistence. In contrast to those in peripheral blood mononuclear cells (PBMCs) following oral dosing, TFV-DP levels in vaginal lymphocytes decreased approximately 7-fold by 3 days, exhibiting a much higher rate of decay. We observed a strong correlation between intracellular TFV-DP in vaginal lymphocytes, in vitro antiviral activity, and in vivo protection, suggesting that TFV-DP above the in vitro IC(95) in vaginal lymphocytes is a good predictor of high efficacy. Data from this model reveal an extended window of protection by TFV gel that supports coitus-independent use. The identification of protective TFV-DP concentrations in vaginal lymphocytes may facilitate the evaluation of improved delivery methods of topical TFV and inform clinical studies.
Figures

Similar articles
-
Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors.J Virol. 2011 Jul;85(13):6610-7. doi: 10.1128/JVI.00311-11. Epub 2011 Apr 27. J Virol. 2011. PMID: 21525346 Free PMC article.
-
Efficacy of topical tenofovir against transmission of a tenofovir-resistant SHIV in macaques.Retrovirology. 2015 Aug 8;12:69. doi: 10.1186/s12977-015-0195-z. Retrovirology. 2015. PMID: 26253002 Free PMC article.
-
Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine.J Virol. 2009 Oct;83(20):10358-65. doi: 10.1128/JVI.01073-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656878 Free PMC article.
-
Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus.Antiviral Res. 2016 Jan;125:63-70. doi: 10.1016/j.antiviral.2015.11.009. Epub 2015 Nov 27. Antiviral Res. 2016. PMID: 26640223 Review.
-
Formulation, pharmacokinetics and pharmacodynamics of topical microbicides.Best Pract Res Clin Obstet Gynaecol. 2012 Aug;26(4):451-62. doi: 10.1016/j.bpobgyn.2012.01.004. Epub 2012 Feb 4. Best Pract Res Clin Obstet Gynaecol. 2012. PMID: 22306523 Free PMC article. Review.
Cited by
-
Translational Models to Predict Target Concentrations for Pre-Exposure Prophylaxis in Women.AIDS Res Hum Retroviruses. 2022 Dec;38(12):909-923. doi: 10.1089/AID.2022.0057. Epub 2022 Oct 25. AIDS Res Hum Retroviruses. 2022. PMID: 36097755 Free PMC article. Review.
-
Safety and pharmacokinetics of intravaginal rings delivering tenofovir in pig-tailed macaques.Antimicrob Agents Chemother. 2012 Nov;56(11):5952-60. doi: 10.1128/AAC.01198-12. Epub 2012 Sep 10. Antimicrob Agents Chemother. 2012. PMID: 22964245 Free PMC article.
-
Topical Inserts: A Versatile Delivery Form for HIV Prevention.Pharmaceutics. 2019 Aug 1;11(8):374. doi: 10.3390/pharmaceutics11080374. Pharmaceutics. 2019. PMID: 31374941 Free PMC article. Review.
-
Effects of gel volume on pharmacokinetics for vaginal and rectal applications of combination DuoGel-IQB4012, a dual chamber-dual drug HIV microbicide gel, in pigtailed macaques.Drug Deliv Transl Res. 2018 Oct;8(5):1180-1190. doi: 10.1007/s13346-018-0538-0. Drug Deliv Transl Res. 2018. PMID: 29761350 Free PMC article.
-
A Phase 1 Clinical Trial to Assess the Safety and Pharmacokinetics of a Tenofovir Alafenamide/Elvitegravir Insert Administered Rectally for HIV Prevention.J Infect Dis. 2024 Sep 23;230(3):696-705. doi: 10.1093/infdis/jiae211. J Infect Dis. 2024. PMID: 38655842 Clinical Trial.
References
-
- Garcia-Lerma JG, et al. 2010. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Sci. Transl. Med. 2:14ra4 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

