The role of MOF in the ionizing radiation response is conserved in Drosophila melanogaster
- PMID: 22072291
- PMCID: PMC4151556
- DOI: 10.1007/s00412-011-0344-7
The role of MOF in the ionizing radiation response is conserved in Drosophila melanogaster
Abstract
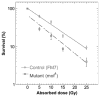
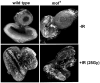
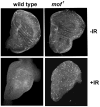
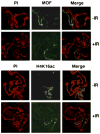
In Drosophila, males absent on the first (MOF) acetylates histone H4 at lysine 16 (H4K16ac). This acetylation mark is highly enriched on the male X chromosome and is required for dosage compensation in Drosophila but not utilized for such in mammals. Recently, we and others reported that mammalian MOF, through H4K16ac, has a critical role at multiple stages in the DNA damage response (DDR) and double-strand break repair pathways. The goal of this study was to test whether mof is similarly required for the response to ionizing radiation (IR) in Drosophila. We report that Drosophila mof mutations in males and females, as well as mof knockdown in SL-2 cells, reduce post-irradiation survival. MOF depletion in SL-2 cells also results in an elevated frequency of metaphases with chromosomal aberrations, suggesting that MOF is involved in DDR. Mutation in Drosophila mof also results in a defective mitotic checkpoint, enhanced apoptosis, and a defective p53 response post-irradiation. In addition, IR exposure enhanced H4K16ac levels in Drosophila as it also does in mammals. These results are the first to demonstrate a requirement for MOF in the whole animal IR response and suggest that the role of MOF in the response to IR is conserved between Drosophila and mammals.
Conflict of interest statement
Figures

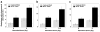


Similar articles
-
The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis.Mol Cell Biol. 2008 Jan;28(1):397-409. doi: 10.1128/MCB.01045-07. Epub 2007 Oct 29. Mol Cell Biol. 2008. PMID: 17967868 Free PMC article.
-
MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair.Mol Cell Biol. 2010 Jul;30(14):3582-95. doi: 10.1128/MCB.01476-09. Epub 2010 May 17. Mol Cell Biol. 2010. PMID: 20479123 Free PMC article.
-
The non-dosage compensated Lsp1alpha gene of Drosophila melanogaster escapes acetylation by MOF in larval fat body nuclei, but is flanked by two dosage compensated genes.BMC Mol Biol. 2007 May 19;8:35. doi: 10.1186/1471-2199-8-35. BMC Mol Biol. 2007. PMID: 17511883 Free PMC article.
-
Males absent on the first (MOF): from flies to humans.Oncogene. 2007 Aug 13;26(37):5385-94. doi: 10.1038/sj.onc.1210607. Oncogene. 2007. PMID: 17694080 Review.
-
A multifaceted role for MOF histone modifying factor in genome maintenance.Mech Ageing Dev. 2017 Jan;161(Pt A):177-180. doi: 10.1016/j.mad.2016.03.012. Epub 2016 Mar 30. Mech Ageing Dev. 2017. PMID: 27038808 Free PMC article. Review.
Cited by
-
Chromatin modifications and the DNA damage response to ionizing radiation.Front Oncol. 2013 Jan 22;2:214. doi: 10.3389/fonc.2012.00214. eCollection 2012. Front Oncol. 2013. PMID: 23346550 Free PMC article.
-
Histone modifications and DNA double-strand break repair after exposure to ionizing radiations.Radiat Res. 2013 Apr;179(4):383-92. doi: 10.1667/RR3308.2. Epub 2013 Feb 1. Radiat Res. 2013. PMID: 23373901 Free PMC article. Review.
-
The Molecular Mechanisms in Senescent Cells Induced by Natural Aging and Ionizing Radiation.Cells. 2024 Mar 21;13(6):550. doi: 10.3390/cells13060550. Cells. 2024. PMID: 38534394 Free PMC article. Review.
-
Heat-induced SIRT1-mediated H4K16ac deacetylation impairs resection and SMARCAD1 recruitment to double strand breaks.iScience. 2022 Mar 23;25(4):104142. doi: 10.1016/j.isci.2022.104142. eCollection 2022 Apr 15. iScience. 2022. PMID: 35434547 Free PMC article.
-
MOF Suppresses Replication Stress and Contributes to Resolution of Stalled Replication Forks.Mol Cell Biol. 2018 Feb 27;38(6):e00484-17. doi: 10.1128/MCB.00484-17. Print 2018 Mar 15. Mol Cell Biol. 2018. PMID: 29298824 Free PMC article.
References
-
- Akhtar A, Becker PB. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol Cell. 2000;5:367–375. - PubMed
-
- Fischle W, Wang Y, Allis CD. Histone and chromatin crosstalk. Curr Opin Cell Biol. 2003;15:172–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

