Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders
- PMID: 22048312
- PMCID: PMC3468323
- DOI: 10.1038/nature10600
Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders
Abstract
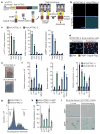
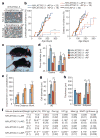
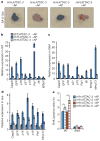
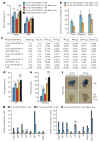
Advanced age is the main risk factor for most chronic diseases and functional deficits in humans, but the fundamental mechanisms that drive ageing remain largely unknown, impeding the development of interventions that might delay or prevent age-related disorders and maximize healthy lifespan. Cellular senescence, which halts the proliferation of damaged or dysfunctional cells, is an important mechanism to constrain the malignant progression of tumour cells. Senescent cells accumulate in various tissues and organs with ageing and have been hypothesized to disrupt tissue structure and function because of the components they secrete. However, whether senescent cells are causally implicated in age-related dysfunction and whether their removal is beneficial has remained unknown. To address these fundamental questions, we made use of a biomarker for senescence, p16(Ink4a), to design a novel transgene, INK-ATTAC, for inducible elimination of p16(Ink4a)-positive senescent cells upon administration of a drug. Here we show that in the BubR1 progeroid mouse background, INK-ATTAC removes p16(Ink4a)-positive senescent cells upon drug treatment. In tissues--such as adipose tissue, skeletal muscle and eye--in which p16(Ink4a) contributes to the acquisition of age-related pathologies, life-long removal of p16(Ink4a)-expressing cells delayed onset of these phenotypes. Furthermore, late-life clearance attenuated progression of already established age-related disorders. These data indicate that cellular senescence is causally implicated in generating age-related phenotypes and that removal of senescent cells can prevent or delay tissue dysfunction and extend healthspan.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Comment in
-
Ageing: Old cells under attack.Nature. 2011 Nov 9;479(7372):186-7. doi: 10.1038/479186a. Nature. 2011. PMID: 22071762 No abstract available.
-
Cellular senescence causes ageing.Nat Rev Mol Cell Biol. 2019 Jul;20(7):388. doi: 10.1038/s41580-019-0128-0. Nat Rev Mol Cell Biol. 2019. PMID: 30962572 No abstract available.
Similar articles
-
Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency.Nat Cell Biol. 2008 Jul;10(7):825-36. doi: 10.1038/ncb1744. Epub 2008 May 30. Nat Cell Biol. 2008. PMID: 18516091 Free PMC article.
-
Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan.Nature. 2016 Feb 11;530(7589):184-9. doi: 10.1038/nature16932. Epub 2016 Feb 3. Nature. 2016. PMID: 26840489 Free PMC article.
-
Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells.Front Endocrinol (Lausanne). 2022 Mar 31;13:869414. doi: 10.3389/fendo.2022.869414. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432205 Free PMC article. Review.
-
Targeted clearance of p21- but not p16-positive senescent cells prevents radiation-induced osteoporosis and increased marrow adiposity.Aging Cell. 2022 May;21(5):e13602. doi: 10.1111/acel.13602. Epub 2022 Apr 1. Aging Cell. 2022. PMID: 35363946 Free PMC article.
-
Adipose tissue inflammation in aging.Exp Gerontol. 2018 May;105:27-31. doi: 10.1016/j.exger.2017.10.014. Epub 2017 Oct 18. Exp Gerontol. 2018. PMID: 29054535 Free PMC article. Review.
Cited by
-
Astroglial asthenia and loss of function, rather than reactivity, contribute to the ageing of the brain.Pflugers Arch. 2021 May;473(5):753-774. doi: 10.1007/s00424-020-02465-3. Epub 2020 Sep 26. Pflugers Arch. 2021. PMID: 32979108 Review.
-
Therapeutic Potential of Senolytics in Cardiovascular Disease.Cardiovasc Drugs Ther. 2022 Feb;36(1):187-196. doi: 10.1007/s10557-020-07075-w. Epub 2020 Sep 26. Cardiovasc Drugs Ther. 2022. PMID: 32979174 Free PMC article. Review.
-
Lipophilic Statins Eliminate Senescent Endothelial Cells by inducing Anoikis-Related Cell Death.Cells. 2023 Dec 14;12(24):2836. doi: 10.3390/cells12242836. Cells. 2023. PMID: 38132158 Free PMC article.
-
Strategies and Challenges in Clinical Trials Targeting Human Aging.J Gerontol A Biol Sci Med Sci. 2016 Nov;71(11):1424-1434. doi: 10.1093/gerona/glw149. Epub 2016 Aug 16. J Gerontol A Biol Sci Med Sci. 2016. PMID: 27535968 Free PMC article.
-
Emerging concepts in drug discovery for cancer therapy.Mol Oncol. 2022 Nov;16(21):3757-3760. doi: 10.1002/1878-0261.13325. Mol Oncol. 2022. PMID: 36321722 Free PMC article. No abstract available.
References
-
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

