Dispersal state of multiwalled carbon nanotubes elicits profibrogenic cellular responses that correlate with fibrogenesis biomarkers and fibrosis in the murine lung
- PMID: 22047207
- PMCID: PMC4136431
- DOI: 10.1021/nn2033055
Dispersal state of multiwalled carbon nanotubes elicits profibrogenic cellular responses that correlate with fibrogenesis biomarkers and fibrosis in the murine lung
Abstract
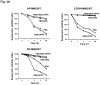
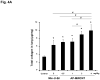
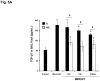
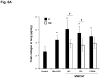
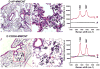
We developed a dispersal method for multiwalled carbon nanotubes (MWCNTs) that allows quantitative assessment of dispersion on profibrogenic responses in tissue culture cells and in mouse lung. We demonstrate that the dispersal of as-prepared (AP), purified (PD), and carboxylated (COOH) MWCNTs by bovine serum albumin (BSA) and dipalmitoylphosphatidylcholine (DPPC) influences TGF-β1, PDGF-AA, and IL-1β production in vitro and in vivo. These biomarkers were chosen based on their synergy in promoting fibrogenesis and cellular communication in the epithelial-mesenchymal cell trophic unit in the lung. The effect of dispersal was most noticeable in AP- and PD-MWCNTs, which are more hydrophobic and unstable in aqueous buffers than hydrophilic COOH-MWCNTs. Well-dispersed AP- and PD-MWCNTs were readily taken up by BEAS-2B, THP-1 cells, and alveolar macrophages (AM) and induced more prominent TGF-β1 and IL-1β production in vitro and TGF-β1, IL-1β, and PDGF-AA production in vivo than nondispersed tubes. Moreover, there was good agreement between the profibrogenic responses in vitro and in vivo as well as the ability of dispersed tubes to generate granulomatous inflammation and fibrosis in airways. Tube dispersal also elicited more robust IL-1β production in THP-1 cells. While COOH-MWCNTs were poorly taken up in BEAS-2B and induced little TGF-β1 production, they were bioprocessed by AM and induced less prominent collagen deposition at sites of nongranulomatous inflammation in the alveolar region. Taken together, these results indicate that the dispersal state of MWCNTs affects profibrogenic cellular responses that correlate with the extent of pulmonary fibrosis and are of potential use to predict pulmonary toxicity.
Figures
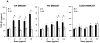
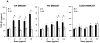
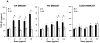
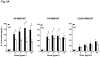
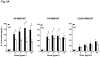





Similar articles
-
Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity.ACS Nano. 2013 Mar 26;7(3):2352-68. doi: 10.1021/nn305567s. Epub 2013 Feb 28. ACS Nano. 2013. PMID: 23414138 Free PMC article.
-
Use of a pro-fibrogenic mechanism-based predictive toxicological approach for tiered testing and decision analysis of carbonaceous nanomaterials.ACS Nano. 2015 Mar 24;9(3):3032-43. doi: 10.1021/nn507243w. Epub 2015 Feb 18. ACS Nano. 2015. PMID: 25646681 Free PMC article.
-
Atomic layer deposition coating of carbon nanotubes with aluminum oxide alters pro-fibrogenic cytokine expression by human mononuclear phagocytes in vitro and reduces lung fibrosis in mice in vivo.PLoS One. 2014 Sep 12;9(9):e106870. doi: 10.1371/journal.pone.0106870. eCollection 2014. PLoS One. 2014. PMID: 25216247 Free PMC article.
-
Predicting pulmonary fibrosis in humans after exposure to multi-walled carbon nanotubes (MWCNTs).Arch Toxicol. 2016 Jul;90(7):1605-22. doi: 10.1007/s00204-016-1742-7. Epub 2016 May 23. Arch Toxicol. 2016. PMID: 27215431 Review.
-
Mechanisms of lung fibrosis induced by carbon nanotubes: towards an Adverse Outcome Pathway (AOP).Part Fibre Toxicol. 2016 Feb 29;13:11. doi: 10.1186/s12989-016-0123-y. Part Fibre Toxicol. 2016. PMID: 26926090 Free PMC article. Review.
Cited by
-
The role of cGAS-STING signaling in pulmonary fibrosis and its therapeutic potential.Front Immunol. 2023 Oct 25;14:1273248. doi: 10.3389/fimmu.2023.1273248. eCollection 2023. Front Immunol. 2023. PMID: 37965345 Free PMC article. Review.
-
Development of Phytochemical Delivery Systems by Nano-Suspension and Nano-Emulsion Techniques.Int J Mol Sci. 2023 Jun 6;24(12):9824. doi: 10.3390/ijms24129824. Int J Mol Sci. 2023. PMID: 37372971 Free PMC article. Review.
-
Toxicity of Carbon-Based Nanomaterials in the Human Lung: A Comparative In-Vitro Study.Tanaffos. 2022 Mar;21(3):391-400. Tanaffos. 2022. PMID: 37025312 Free PMC article.
-
CeO2 nanoparticles induce pulmonary fibrosis via activating S1P pathway as revealed by metabolomics.Nano Today. 2022 Aug;45:101559. doi: 10.1016/j.nantod.2022.101559. Epub 2022 Jul 23. Nano Today. 2022. PMID: 36910843 Free PMC article.
-
The State of the Art and Challenges of In Vitro Methods for Human Hazard Assessment of Nanomaterials in the Context of Safe-by-Design.Nanomaterials (Basel). 2023 Jan 24;13(3):472. doi: 10.3390/nano13030472. Nanomaterials (Basel). 2023. PMID: 36770432 Free PMC article. Review.
References
-
- Baughman RH, Zakhidov AA, de Heer WA. Carbon Nanotubes - the Route Toward Applications. Science. 2002;297:787–792. - PubMed
-
- Iijima S. Helical Microtubules of Graphitic Carbon. Nature. 1991;354:56–58.
-
- Nel A, Xia T, Madler L, Li N. Toxic Potential of Materials at the Nanolevel. Science. 2006;311:622–627. - PubMed
-
- Kostarelos K, Bianco A, Prato M. Promises, Facts and Challenges for Carbon Nanotubes in Imaging and Therapeutics. Nat Nanotechnol. 2009;4:627–633. - PubMed
-
- Lin Y, Taylor S, Li HP, Fernando KAS, Qu LW, Wang W, Gu LR, Zhou B, Sun YP. Advances Toward Bioapplications of Carbon Nanotubes. J Mater Chem. 2004;14:527–541.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

