Generation and characterization of transgenic mice expressing mitochondrial targeted red fluorescent protein selectively in neurons: modeling mitochondriopathy in excitotoxicity and amyotrophic lateral sclerosis
- PMID: 22047141
- PMCID: PMC3227596
- DOI: 10.1186/1750-1326-6-75
Generation and characterization of transgenic mice expressing mitochondrial targeted red fluorescent protein selectively in neurons: modeling mitochondriopathy in excitotoxicity and amyotrophic lateral sclerosis
Abstract
Background: Mitochondria have roles or appear to have roles in the pathogenesis of several chronic age-related and acute neurological disorders, including Charcot-Marie-Tooth disease, amyotrophic lateral sclerosis, Parkinson's disease, and cerebral ischemia, and could be critical targets for development of rational mechanism-based, disease-modifying therapeutics for treating these disorders effectively. A deeper understanding of neural tissue mitochondria pathobiologies as definitive mediators of neural injury, disease, and cell death merits further study, and the development of additional tools to study neural mitochondria will help achieve this unmet need.
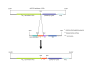
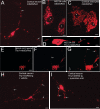
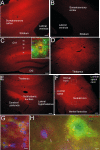
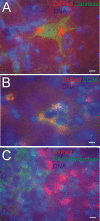
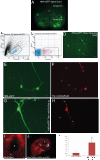
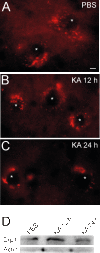
Results: We created transgenic mice that express the coral (Discosoma sp.) red fluorescent protein DsRed2 specifically in mitochondria of neurons using a construct engineered with a Thy1 promoter, specific for neuron expression, to drive expression of a fusion protein of DsRed2 with a mitochondrial targeting sequence. The biochemical and histological characterization of these mice shows the expression of mitochondrial-targeted DsRed2 to be specific for mitochondria and concentrated in distinct CNS regions, including cerebral cortex, hippocampus, thalamus, brainstem, and spinal cord. Red fluorescent mitochondria were visualized in cerebral cortical and hippocampal pyramidal neurons, ventrobasal thalamic neurons, subthalamic neurons, and spinal motor neurons. For the purpose of proof of principle application, these mice were used in excitotoxicity paradigms and double transgenic mice were generated by crossing Thy1-mitoDsRed2 mice with transgenic mice expressing enhanced-GFP (eGFP) under the control of the Hlxb9 promoter that drives eGFP expression specifically in motor neurons and by crossing Thy1-mitoDsRed2 mice to amyotrophic lateral sclerosis (ALS) mice expressing human mutant superoxide dismutase-1.
Conclusions: These novel transgenic mice will be a useful tool for better understanding the biology of mitochondria in mouse and cellular models of human neurological disorders as exemplified by the mitochondrial swelling and fission seen in excitotoxicity and mouse ALS.
Figures

Similar articles
-
Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death.J Comp Neurol. 2007 Jan 1;500(1):20-46. doi: 10.1002/cne.21160. J Comp Neurol. 2007. PMID: 17099894
-
Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1.Neurobiol Dis. 2000 Dec;7(6 Pt B):623-43. doi: 10.1006/nbdi.2000.0299. Neurobiol Dis. 2000. PMID: 11114261
-
Transgenic mice with human mutant genes causing Parkinson's disease and amyotrophic lateral sclerosis provide common insight into mechanisms of motor neuron selective vulnerability to degeneration.Rev Neurosci. 2007;18(2):115-36. doi: 10.1515/revneuro.2007.18.2.115. Rev Neurosci. 2007. PMID: 17593875 Review.
-
The mitochondrial calcium regulator cyclophilin D is an essential component of oestrogen-mediated neuroprotection in amyotrophic lateral sclerosis.Brain. 2012 Sep;135(Pt 9):2865-74. doi: 10.1093/brain/aws208. Brain. 2012. PMID: 22961554 Free PMC article.
-
Mitochondrial dysfunction and its role in motor neuron degeneration in ALS.Mitochondrion. 2005 Apr;5(2):77-87. doi: 10.1016/j.mito.2005.01.002. Mitochondrion. 2005. PMID: 16050975 Review.
Cited by
-
DNA Damage Response and Repair, DNA Methylation, and Cell Death in Human Neurons and Experimental Animal Neurons Are Different.J Neuropathol Exp Neurol. 2018 Jul 1;77(7):636-655. doi: 10.1093/jnen/nly040. J Neuropathol Exp Neurol. 2018. PMID: 29788379 Free PMC article.
-
The dynamics of the mitochondrial organelle as a potential therapeutic target.J Cereb Blood Flow Metab. 2013 Jan;33(1):22-32. doi: 10.1038/jcbfm.2012.158. Epub 2012 Oct 24. J Cereb Blood Flow Metab. 2013. PMID: 23093069 Free PMC article. Review.
-
Transgenic overexpression of polysialyltransferase ST8SiaIV under the control of a neuron-specific promoter does not affect brain development but impairs exploratory behavior.Glycobiology. 2019 Aug 20;29(9):657-668. doi: 10.1093/glycob/cwz040. Glycobiology. 2019. PMID: 31147692 Free PMC article.
-
DNA Damage Response and DNA Repair in Skeletal Myocytes From a Mouse Model of Spinal Muscular Atrophy.J Neuropathol Exp Neurol. 2016 Sep;75(9):889-902. doi: 10.1093/jnen/nlw064. Epub 2016 Jul 24. J Neuropathol Exp Neurol. 2016. PMID: 27452406 Free PMC article.
-
Fiber deprivation and microbiome-borne curli shift gut bacterial populations and accelerate disease in a mouse model of Parkinson's disease.Cell Rep. 2023 Sep 26;42(9):113071. doi: 10.1016/j.celrep.2023.113071. Epub 2023 Sep 8. Cell Rep. 2023. PMID: 37676767 Free PMC article.
References
-
- Zuchner S, Meriyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J. et al.Mutation in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Gen. 2004;36:449–451. - PubMed
-
- Smith MA, Perry G. Free radical damage, iron, and Alzheimer's disease. J Neurol Sci. 1995;134(Suppl):92–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

