Nutrition, epigenetics, and metabolic syndrome
- PMID: 22044276
- PMCID: PMC3353821
- DOI: 10.1089/ars.2011.4381
Nutrition, epigenetics, and metabolic syndrome
Abstract
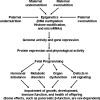
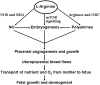
Significance: Epidemiological and animal studies have demonstrated a close link between maternal nutrition and chronic metabolic disease in children and adults. Compelling experimental results also indicate that adverse effects of intrauterine growth restriction on offspring can be carried forward to subsequent generations through covalent modifications of DNA and core histones.
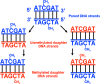
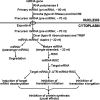
Recent advances: DNA methylation is catalyzed by S-adenosylmethionine-dependent DNA methyltransferases. Methylation, demethylation, acetylation, and deacetylation of histone proteins are performed by histone methyltransferase, histone demethylase, histone acetyltransferase, and histone deacetyltransferase, respectively. Histone activities are also influenced by phosphorylation, ubiquitination, ADP-ribosylation, sumoylation, and glycosylation. Metabolism of amino acids (glycine, histidine, methionine, and serine) and vitamins (B6, B12, and folate) plays a key role in provision of methyl donors for DNA and protein methylation.
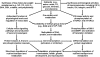
Critical issues: Disruption of epigenetic mechanisms can result in oxidative stress, obesity, insulin resistance, diabetes, and vascular dysfunction in animals and humans. Despite a recognized role for epigenetics in fetal programming of metabolic syndrome, research on therapies is still in its infancy. Possible interventions include: 1) inhibition of DNA methylation, histone deacetylation, and microRNA expression; 2) targeting epigenetically disturbed metabolic pathways; and 3) dietary supplementation with functional amino acids, vitamins, and phytochemicals.
Future directions: Much work is needed with animal models to understand the basic mechanisms responsible for the roles of specific nutrients in fetal and neonatal programming. Such new knowledge is crucial to design effective therapeutic strategies for preventing and treating metabolic abnormalities in offspring born to mothers with a previous experience of malnutrition.
Figures




Similar articles
-
Nutritional epigenetics with a focus on amino acids: implications for the development and treatment of metabolic syndrome.J Nutr Biochem. 2016 Jan;27:1-8. doi: 10.1016/j.jnutbio.2015.08.003. Epub 2015 Aug 10. J Nutr Biochem. 2016. PMID: 26427799 Review.
-
Gestational Nutrition as a Predisposing Factor to Obesity Onset in Offspring: Role for Involvement of Epigenetic Mechanism.Niger J Physiol Sci. 2022 Jun 30;37(1):1-7. doi: 10.54548/njps.v37i1.1. Niger J Physiol Sci. 2022. PMID: 35947841 Review.
-
Interplay between early-life malnutrition, epigenetic modulation of the immune function and liver diseases.Nutr Res Rev. 2019 Jun;32(1):128-145. doi: 10.1017/S0954422418000239. Epub 2019 Feb 1. Nutr Res Rev. 2019. PMID: 30707092 Review.
-
The Effects of Maternal and Postnatal Dietary Methyl Nutrients on Epigenetic Changes that Lead to Non-Communicable Diseases in Adulthood.Int J Mol Sci. 2020 May 6;21(9):3290. doi: 10.3390/ijms21093290. Int J Mol Sci. 2020. PMID: 32384688 Free PMC article. Review.
-
Epigenomics, gestational programming and risk of metabolic syndrome.Int J Obes (Lond). 2015 Apr;39(4):633-41. doi: 10.1038/ijo.2015.13. Epub 2015 Feb 2. Int J Obes (Lond). 2015. PMID: 25640766 Review.
Cited by
-
From Mice to Men: research models of developmental programming.J Dev Orig Health Dis. 2013 Feb;4(1):3-9. doi: 10.1017/S2040174412000487. J Dev Orig Health Dis. 2013. PMID: 23525085 Free PMC article. Review.
-
Zebrafish as a model to study the role of DNA methylation in environmental toxicology.Environ Sci Pollut Res Int. 2015 Nov;22(21):16262-76. doi: 10.1007/s11356-014-3466-7. Epub 2014 Aug 31. Environ Sci Pollut Res Int. 2015. PMID: 25172464 Review.
-
Metabolic, Epigenetic, and Transgenerational Effects of Gut Bacterial Choline Consumption.Cell Host Microbe. 2017 Sep 13;22(3):279-290.e7. doi: 10.1016/j.chom.2017.07.021. Epub 2017 Aug 24. Cell Host Microbe. 2017. PMID: 28844887 Free PMC article.
-
Metabolic Syndrome Programming and Reprogramming: Mechanistic Aspects of Oxidative Stress.Antioxidants (Basel). 2022 Oct 26;11(11):2108. doi: 10.3390/antiox11112108. Antioxidants (Basel). 2022. PMID: 36358480 Free PMC article. Review.
-
The Importance of Metabolic and Environmental Factors in the Occurrence of Oxidative Stress during Pregnancy.Int J Mol Sci. 2023 Jul 26;24(15):11964. doi: 10.3390/ijms241511964. Int J Mol Sci. 2023. PMID: 37569340 Free PMC article. Review.
References
-
- Ainge H. Thompson C. Ozanne SE. Rooney KB. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes. 2011;35:325–335. - PubMed
-
- Ambros V. microRNAs: Tiny regulators with great potential. Cell. 2001;107:823–826. - PubMed
-
- Anderson CM. Lopez F. Zimmer A. Benoit JN. Placental insufficiency leads to developmental hypertension and mesenteric artery dysfunction in two generations of Sprague-Dawley rat offspring. Biol Reprod. 2006;74:538–544. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

