The blood-testis barrier and its implications for male contraception
- PMID: 22039149
- PMCID: PMC3250082
- DOI: 10.1124/pr.110.002790
The blood-testis barrier and its implications for male contraception
Abstract
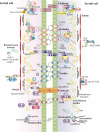
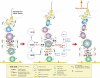
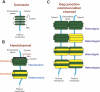
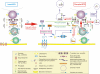
The blood-testis barrier (BTB) is one of the tightest blood-tissue barriers in the mammalian body. It divides the seminiferous epithelium into the basal and the apical (adluminal) compartments. Meiosis I and II, spermiogenesis, and spermiation all take place in a specialized microenvironment behind the BTB in the apical compartment, but spermatogonial renewal and differentiation and cell cycle progression up to the preleptotene spermatocyte stage take place outside of the BTB in the basal compartment of the epithelium. However, the BTB is not a static ultrastructure. Instead, it undergoes extensive restructuring during the seminiferous epithelial cycle of spermatogenesis at stage VIII to allow the transit of preleptotene spermatocytes at the BTB. Yet the immunological barrier conferred by the BTB cannot be compromised, even transiently, during the epithelial cycle to avoid the production of antibodies against meiotic and postmeiotic germ cells. Studies have demonstrated that some unlikely partners, namely adhesion protein complexes (e.g., occludin-ZO-1, N-cadherin-β-catenin, claudin-5-ZO-1), steroids (e.g., testosterone, estradiol-17β), nonreceptor protein kinases (e.g., focal adhesion kinase, c-Src, c-Yes), polarity proteins (e.g., PAR6, Cdc42, 14-3-3), endocytic vesicle proteins (e.g., clathrin, caveolin, dynamin 2), and actin regulatory proteins (e.g., Eps8, Arp2/3 complex), are working together, apparently under the overall influence of cytokines (e.g., transforming growth factor-β3, tumor necrosis factor-α, interleukin-1α). In short, a "new" BTB is created behind spermatocytes in transit while the "old" BTB above transiting cells undergoes timely degeneration, so that the immunological barrier can be maintained while spermatocytes are traversing the BTB. We also discuss recent findings regarding the molecular mechanisms by which environmental toxicants (e.g., cadmium, bisphenol A) induce testicular injury via their initial actions at the BTB to elicit subsequent damage to germ-cell adhesion, thereby leading to germ-cell loss, reduced sperm count, and male infertility or subfertility. Moreover, we also critically evaluate findings in the field regarding studies on drug transporters in the testis and discuss how these influx and efflux pumps regulate the entry of potential nonhormonal male contraceptives to the apical compartment to exert their effects. Collectively, these findings illustrate multiple potential targets are present at the BTB for innovative contraceptive development and for better delivery of drugs to alleviate toxicant-induced reproductive dysfunction in men.
Figures




Similar articles
-
The apical ectoplasmic specialization-blood-testis barrier functional axis is a novel target for male contraception.Adv Exp Med Biol. 2012;763:334-355. doi: 10.1007/978-1-4614-4711-5_17. Adv Exp Med Biol. 2012. PMID: 23397633 Free PMC article.
-
P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK).Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):19623-8. doi: 10.1073/pnas.1111414108. Epub 2011 Nov 21. Proc Natl Acad Sci U S A. 2011. PMID: 22106313 Free PMC article.
-
Regulation of blood-testis barrier dynamics by TGF-beta3 is a Cdc42-dependent protein trafficking event.Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11399-404. doi: 10.1073/pnas.1001077107. Epub 2010 Jun 7. Proc Natl Acad Sci U S A. 2010. PMID: 20534521 Free PMC article.
-
Drug transporters, the blood-testis barrier, and spermatogenesis.J Endocrinol. 2011 Mar;208(3):207-23. doi: 10.1677/JOE-10-0363. Epub 2010 Dec 6. J Endocrinol. 2011. PMID: 21134990 Free PMC article. Review.
-
Effective Delivery of Male Contraceptives Behind the Blood-Testis Barrier (BTB) - Lesson from Adjudin.Curr Med Chem. 2016;23(7):701-13. doi: 10.2174/0929867323666160112122724. Curr Med Chem. 2016. PMID: 26758796 Free PMC article. Review.
Cited by
-
Secreted Frizzled-related protein 1 (sFRP1) regulates spermatid adhesion in the testis via dephosphorylation of focal adhesion kinase and the nectin-3 adhesion protein complex.FASEB J. 2013 Feb;27(2):464-77. doi: 10.1096/fj.12-212514. Epub 2012 Oct 16. FASEB J. 2013. PMID: 23073828 Free PMC article.
-
Advantages of Curcuma longa in preventing male infertility caused by thioacetamide.Open Vet J. 2024 Jul;14(7):1585-1595. doi: 10.5455/OVJ.2024.v14.i7.8. Epub 2024 Jul 31. Open Vet J. 2024. PMID: 39175971 Free PMC article.
-
mTORC1/C2 regulate spermatogenesis in Eriocheir sinensis via alterations in the actin filament network and cell junctions.Cell Tissue Res. 2022 Nov;390(2):293-313. doi: 10.1007/s00441-022-03680-3. Epub 2022 Aug 31. Cell Tissue Res. 2022. PMID: 36044078
-
The Protective Role of L-Cysteine in the Regulation of Blood-Testis Barrier Functions-A Brief Review.Genes (Basel). 2024 Sep 12;15(9):1201. doi: 10.3390/genes15091201. Genes (Basel). 2024. PMID: 39336792 Free PMC article. Review.
-
Simian immunodeficiency virus infection and immune responses in the pig-tailed macaque testis.J Leukoc Biol. 2015 Mar;97(3):599-609. doi: 10.1189/jlb.4A0914-438R. Epub 2015 Jan 20. J Leukoc Biol. 2015. PMID: 25605872 Free PMC article.
References
-
- Ahmed S, Goh WI, Bu W. (2010) I-BAR domains, IRSp53 and filopodium formation. Semin Cell Dev Biol 21:350–356 - PubMed
-
- Agency for Toxic Substances and Disease Registry (2008) Cadmium toxicity: ATSDR Case Studies in Environmental Medicine. Agency for Toxic Substances and Disease Registry, Atlanta, GA: Available at: http://www.atsdr.cdc.gov/csem/cadmium/docs/cadmium.pdf - PubMed
-
- Aitken RJ, Findlay JK, Hutt KJ, Kerr JB. (2011) Apoptosis in the germ line. Reproduction 141:139–150 - PubMed
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. (2002) Molecular Biology of the Cell, 4th ed, Garland Science, New York
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
