A unified model of mammalian BCL-2 protein family interactions at the mitochondria
- PMID: 22036586
- PMCID: PMC3221787
- DOI: 10.1016/j.molcel.2011.10.001
A unified model of mammalian BCL-2 protein family interactions at the mitochondria
Abstract
During apoptosis, the BCL-2 protein family controls mitochondrial outer membrane permeabilization (MOMP), but the dynamics of this regulation remain controversial. We employed chimeric proteins composed of exogenous BH3 domains inserted into a tBID backbone that can activate the proapoptotic effectors BAX and BAK to permeabilize membranes without being universally sequestered by all antiapoptotic BCL-2 proteins. We thus identified two "modes" whereby prosurvival BCL-2 proteins can block MOMP, by sequestering direct-activator BH3-only proteins ("MODE 1") or by binding active BAX and BAK ("MODE 2"). Notably, we found that MODE 1 sequestration is less efficient and more easily derepressed to promote MOMP than MODE 2. Further, MODE 2 sequestration prevents mitochondrial fusion. We provide a unified model of BCL-2 family function that helps to explain otherwise paradoxical observations relating to MOMP, apoptosis, and mitochondrial dynamics.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
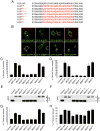
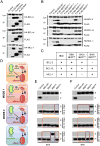
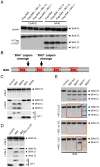

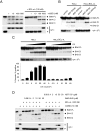
Comment in
-
Staying alive: defensive strategies in the BCL-2 family playbook.Mol Cell. 2011 Nov 18;44(4):509-10. doi: 10.1016/j.molcel.2011.11.002. Mol Cell. 2011. PMID: 22099298
Similar articles
-
Distinct lipid effects on tBid and Bim activation of membrane permeabilization by pro-apoptotic Bax.Biochem J. 2015 May 1;467(3):495-505. doi: 10.1042/BJ20141291. Biochem J. 2015. PMID: 25714678
-
BH3 domains other than Bim and Bid can directly activate Bax/Bak.J Biol Chem. 2011 Jan 7;286(1):491-501. doi: 10.1074/jbc.M110.167148. Epub 2010 Nov 1. J Biol Chem. 2011. PMID: 21041309 Free PMC article.
-
The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner.J Biol Chem. 2011 Mar 18;286(11):9382-92. doi: 10.1074/jbc.M110.203638. Epub 2010 Dec 9. J Biol Chem. 2011. PMID: 21148306 Free PMC article.
-
How do BCL-2 proteins induce mitochondrial outer membrane permeabilization?Trends Cell Biol. 2008 Apr;18(4):157-64. doi: 10.1016/j.tcb.2008.01.007. Epub 2008 Mar 7. Trends Cell Biol. 2008. PMID: 18314333 Free PMC article. Review.
-
BCL-2 proteins and apoptosis: Recent insights and unknowns.Biochem Biophys Res Commun. 2018 May 27;500(1):26-34. doi: 10.1016/j.bbrc.2017.06.190. Epub 2017 Jul 1. Biochem Biophys Res Commun. 2018. PMID: 28676391 Review.
Cited by
-
Your neighbours matter - non-autonomous control of apoptosis in development and disease.Cell Death Differ. 2016 Jul;23(7):1110-8. doi: 10.1038/cdd.2016.41. Epub 2016 May 13. Cell Death Differ. 2016. PMID: 27177021 Free PMC article. Review.
-
Programming cancer cells for high expression levels of Mcl1.EMBO Rep. 2013 Apr;14(4):328-36. doi: 10.1038/embor.2013.20. Epub 2013 Mar 12. EMBO Rep. 2013. PMID: 23478333 Free PMC article. Review.
-
Precision Targeting of BFL-1/A1 and an ATM Co-dependency in Human Cancer.Cell Rep. 2018 Sep 25;24(13):3393-3403.e5. doi: 10.1016/j.celrep.2018.08.089. Cell Rep. 2018. PMID: 30257201 Free PMC article.
-
BAX, BAK, and BOK: A Coming of Age for the BCL-2 Family Effector Proteins.Cold Spring Harb Perspect Biol. 2020 Apr 1;12(4):a036319. doi: 10.1101/cshperspect.a036319. Cold Spring Harb Perspect Biol. 2020. PMID: 31570337 Free PMC article. Review.
-
Disruption of mitochondrial quality control genes promotes caspase-resistant cell survival following apoptotic stimuli.J Biol Chem. 2022 Apr;298(4):101835. doi: 10.1016/j.jbc.2022.101835. Epub 2022 Mar 16. J Biol Chem. 2022. PMID: 35304098 Free PMC article.
References
-
- Adler J, Parmryd I. Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander’s overlap coefficient. Cytometry A. 2010;77:733–742. - PubMed
-
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. - PubMed
-
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DCS. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Molecular Cell. 2005;17:393–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

