Microglia and memory: modulation by early-life infection
- PMID: 22031897
- PMCID: PMC3224817
- DOI: 10.1523/JNEUROSCI.3688-11.2011
Microglia and memory: modulation by early-life infection
Abstract
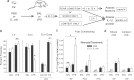
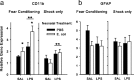
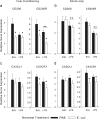
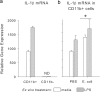
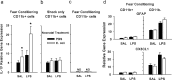
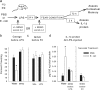
The proinflammatory cytokine interleukin-1β (IL-1β) is critical for normal hippocampus (HP)-dependent cognition, whereas high levels can disrupt memory and are implicated in neurodegeneration. However, the cellular source of IL-1β during learning has not been shown, and little is known about the risk factors leading to cytokine dysregulation within the HP. We have reported that neonatal bacterial infection in rats leads to marked HP-dependent memory deficits in adulthood. However, deficits are only observed if unmasked by a subsequent immune challenge [lipopolysaccharide (LPS)] around the time of learning. These data implicate a long-term change within the immune system that, upon activation with the "second hit," LPS, acutely impacts the neural processes underlying memory. Indeed, inhibiting brain IL-1β before the LPS challenge prevents memory impairment in neonatally infected (NI) rats. We aimed to determine the cellular source of IL-1β during normal learning and thereby lend insight into the mechanism by which this cytokine is enduringly altered by early-life infection. We show for the first time that CD11b(+) enriched cells are the source of IL-1β during normal HP-dependent learning. CD11b(+) cells from NI rats are functionally sensitized within the adult HP and produce exaggerated IL-1β ex vivo compared with controls. However, an exaggerated IL-1β response in vivo requires LPS before learning. Moreover, preventing microglial activation during learning prevents memory impairment in NI rats, even following an LPS challenge. Thus, early-life events can significantly modulate normal learning-dependent cytokine activity within the HP, via a specific, enduring impact on brain microglial function.
Figures

Similar articles
-
Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge.Brain Behav Immun. 2010 Oct;24(7):1190-201. doi: 10.1016/j.bbi.2010.05.011. Epub 2010 Jun 4. Brain Behav Immun. 2010. PMID: 20570721 Free PMC article.
-
Neonatal infection produces significant changes in immune function with no associated learning deficits in juvenile rats.Dev Neurobiol. 2017 Oct;77(10):1221-1236. doi: 10.1002/dneu.22512. Epub 2017 Jul 25. Dev Neurobiol. 2017. PMID: 28719141 Free PMC article.
-
Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines.Brain Behav Immun. 2009 Mar;23(3):309-17. doi: 10.1016/j.bbi.2008.09.002. Epub 2008 Sep 12. Brain Behav Immun. 2009. PMID: 18814846 Free PMC article.
-
Frank A. Beach award: programming of neuroendocrine function by early-life experience: a critical role for the immune system.Horm Behav. 2013 May;63(5):684-91. doi: 10.1016/j.yhbeh.2013.02.017. Epub 2013 Mar 6. Horm Behav. 2013. PMID: 23474365 Free PMC article. Review.
-
The Perfect Cytokine Storm: How Peripheral Immune Challenges Impact Brain Plasticity & Memory Function in Aging.Brain Plast. 2021 Aug 23;7(1):47-60. doi: 10.3233/BPL-210127. eCollection 2021. Brain Plast. 2021. PMID: 34631420 Free PMC article. Review.
Cited by
-
Sex differences in microglial colonization of the developing rat brain.J Neurochem. 2012 Mar;120(6):948-63. doi: 10.1111/j.1471-4159.2011.07630.x. Epub 2012 Feb 9. J Neurochem. 2012. PMID: 22182318 Free PMC article.
-
Inflammation of the Embryonic Choroid Plexus Barrier following Maternal Immune Activation.Dev Cell. 2020 Dec 7;55(5):617-628.e6. doi: 10.1016/j.devcel.2020.09.020. Epub 2020 Oct 9. Dev Cell. 2020. PMID: 33038331 Free PMC article.
-
The Long and the Short of it: Gene and Environment Interactions During Early Cortical Development and Consequences for Long-Term Neurological Disease.Front Psychiatry. 2012 Jun 12;3:50. doi: 10.3389/fpsyt.2012.00050. eCollection 2012. Front Psychiatry. 2012. PMID: 22701439 Free PMC article.
-
Sex Differences in Neurodevelopmental Disorders: A Key Role for the Immune System.Curr Top Behav Neurosci. 2023;62:165-206. doi: 10.1007/7854_2022_308. Curr Top Behav Neurosci. 2023. PMID: 35435643 Free PMC article.
-
Neonatal infection leads to increased susceptibility to Aβ oligomer-induced brain inflammation, synapse loss and cognitive impairment in mice.Cell Death Dis. 2019 Apr 11;10(4):323. doi: 10.1038/s41419-019-1529-x. Cell Death Dis. 2019. PMID: 30975983 Free PMC article.
References
-
- Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–290. - PubMed
-
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. - PubMed
-
- Ben Menachem-Zidon O, Avital A, Ben-Menahem Y, Goshen I, Kreisel T, Shmueli EM, Segal M, Ben Hur T, Yirmiya R. Astrocytes support hippocampal-dependent memory and long-term potentiation via interleukin-1 signaling. Brain Behav Immun. 2011;25:1008–1016. - PubMed
-
- Bennet L, Gunn A. The fetal origins of adult mental illness. In: Wintour-Coghlan M, Owens J, editors. Early life origins of health and disease. Advances in experimental medicine and biology. New York: Springer; 2006. pp. 204–211.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

