Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles
- PMID: 22024752
- PMCID: PMC3288016
- DOI: 10.4161/auto.7.12.17877
Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles
Abstract
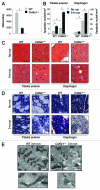
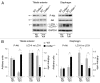
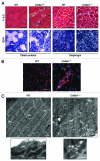
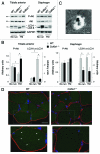
Autophagy is a catabolic process that provides the degradation of altered/damaged organelles through the fusion between autophagosomes and lysosomes. Proper regulation of the autophagic flux is fundamental for the homeostasis of skeletal muscles in physiological conditions and in response to stress. Defective as well as excessive autophagy is detrimental for muscle health and has a pathogenic role in several forms of muscle diseases. Recently, we found that defective activation of the autophagic machinery plays a key role in the pathogenesis of muscular dystrophies linked to collagen VI. Impairment of the autophagic flux in collagen VI null (Col6a1–/–) mice causes accumulation of dysfunctional mitochondria and altered sarcoplasmic reticulum, leading to apoptosis and degeneration of muscle fibers. Here we show that physical exercise activates autophagy in skeletal muscles. Notably, physical training exacerbated the dystrophic phenotype of Col6a1–/– mice, where autophagy flux is compromised. Autophagy was not induced in Col6a1–/– muscles after either acute or prolonged exercise, and this led to a marked increase of muscle wasting and apoptosis. These findings indicate that proper activation of autophagy is important for muscle homeostasis during physical activity.
Figures
Comment in
-
Activation of autophagy is required for muscle homeostasis during physical exercise.Autophagy. 2011 Dec;7(12):1405-6. doi: 10.4161/auto.7.12.18315. Autophagy. 2011. PMID: 22082869 Free PMC article.
Similar articles
-
Activation of autophagy is required for muscle homeostasis during physical exercise.Autophagy. 2011 Dec;7(12):1405-6. doi: 10.4161/auto.7.12.18315. Autophagy. 2011. PMID: 22082869 Free PMC article.
-
Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration.Nat Med. 2010 Nov;16(11):1313-20. doi: 10.1038/nm.2247. Epub 2010 Oct 31. Nat Med. 2010. PMID: 21037586
-
Reactivation of autophagy by spermidine ameliorates the myopathic defects of collagen VI-null mice.Autophagy. 2015;11(12):2142-52. doi: 10.1080/15548627.2015.1108508. Autophagy. 2015. PMID: 26565691 Free PMC article.
-
Mitochondrial dysfunction and defective autophagy in the pathogenesis of collagen VI muscular dystrophies.Cold Spring Harb Perspect Biol. 2013 May 1;5(5):a011387. doi: 10.1101/cshperspect.a011387. Cold Spring Harb Perspect Biol. 2013. PMID: 23580791 Free PMC article. Review.
-
Autophagy in the mesh of collagen VI.Matrix Biol. 2021 Jun;100-101:162-172. doi: 10.1016/j.matbio.2020.12.004. Epub 2020 Dec 26. Matrix Biol. 2021. PMID: 33373668 Review.
Cited by
-
Exercise sustains the hallmarks of health.J Sport Health Sci. 2023 Jan;12(1):8-35. doi: 10.1016/j.jshs.2022.10.003. Epub 2022 Oct 29. J Sport Health Sci. 2023. PMID: 36374766 Free PMC article. Review.
-
Mitochondrial quality control: Easy come, easy go.Biochim Biophys Acta. 2015 Oct;1853(10 Pt B):2802-11. doi: 10.1016/j.bbamcr.2014.12.041. Epub 2015 Jan 14. Biochim Biophys Acta. 2015. PMID: 25596427 Free PMC article. Review.
-
Cellular and molecular mechanisms of muscle atrophy.Dis Model Mech. 2013 Jan;6(1):25-39. doi: 10.1242/dmm.010389. Dis Model Mech. 2013. PMID: 23268536 Free PMC article. Review.
-
Heat shock response and autophagy--cooperation and control.Autophagy. 2015;11(2):200-13. doi: 10.1080/15548627.2015.1009776. Autophagy. 2015. PMID: 25714619 Free PMC article. Review.
-
Periodized low protein-high carbohydrate diet confers potent, but transient, metabolic improvements.Mol Metab. 2018 Nov;17:112-121. doi: 10.1016/j.molmet.2018.08.008. Epub 2018 Aug 28. Mol Metab. 2018. PMID: 30193785 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
