The use of chemokine-releasing tissue engineering scaffolds in a model of inflammatory response-mediated melanoma cancer metastasis
- PMID: 22019117
- PMCID: PMC3220744
- DOI: 10.1016/j.biomaterials.2011.10.002
The use of chemokine-releasing tissue engineering scaffolds in a model of inflammatory response-mediated melanoma cancer metastasis
Abstract
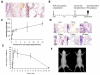
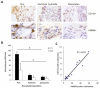
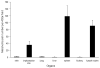
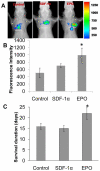
Inflammatory responses and associated products have been implicated in cancer metastasis. However, the relationship between these two processes is uncertain due to the lack of a suitable model. Taking advantage of localized and controllable inflammatory responses induced by biomaterial implantation and the capability of tissue scaffolds to release a wide variety of chemokines, we report a novel system for studying the molecular mechanisms of inflammation-mediated cancer metastasis. The animal model is comprised of an initial subcutaneous implantation of biomaterial microspheres which prompt localized inflammatory responses, followed by the transplantation of metastatic cancer cells into the peritoneal cavity or blood circulation. Histological results demonstrated that substantial numbers of B16F10 cells were recruited to the site nearby biomaterial implants. There was a strong correlation between the degree of biomaterial-mediated inflammatory responses and number of recruited cancer cells. Inflammation-mediated cancer cell migration was inhibited by small molecule inhibitors of CXCR4 but not by neutralizing antibody against CCL21. Using chemokine-releasing scaffolds, further studies were carried out to explore the possibility of enhancing cancer cell recruitment. Interestingly, erythropoietin (EPO) releasing scaffolds, but not stromal cell-derived factor-1α-releasing scaffolds, were found to accumulate substantially more melanoma cells than controls. Rather unexpectedly, perhaps by indirectly reducing circulating cancer cells, mice implanted with EPO-releasing scaffolds had ~30% longer life span than other groups. These results suggest that chemokine-releasing scaffolds may potentially function as implantable cancer traps and serve as powerful tools for studying cancer distraction and even selective annihilation of circulating metastatic cancer cells.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
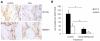
Similar articles
-
In vivo biocompatibility assessment of poly (ether imide) electrospun scaffolds.J Tissue Eng Regen Med. 2017 Apr;11(4):1034-1044. doi: 10.1002/term.2002. Epub 2015 Feb 25. J Tissue Eng Regen Med. 2017. PMID: 25712330
-
The effect of incorporation of SDF-1alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response.Biomaterials. 2010 May;31(14):3997-4008. doi: 10.1016/j.biomaterials.2010.01.144. Epub 2010 Feb 24. Biomaterials. 2010. PMID: 20185171 Free PMC article.
-
Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering.Acta Biomater. 2019 Apr 1;88:301-313. doi: 10.1016/j.actbio.2019.02.044. Epub 2019 Feb 27. Acta Biomater. 2019. PMID: 30825604
-
The good and the bad of chemokines/chemokine receptors in melanoma.Pigment Cell Melanoma Res. 2009 Apr;22(2):175-86. doi: 10.1111/j.1755-148X.2009.00554.x. Epub 2009 Feb 14. Pigment Cell Melanoma Res. 2009. PMID: 19222802 Free PMC article. Review.
-
Silk scaffolds in bone tissue engineering: An overview.Acta Biomater. 2017 Nov;63:1-17. doi: 10.1016/j.actbio.2017.09.027. Epub 2017 Sep 20. Acta Biomater. 2017. PMID: 28941652 Review.
Cited by
-
Preparation of a novel injectable in situ-gelling nanoparticle with applications in controlled protein release and cancer cell entrapment.RSC Adv. 2018 Oct 9;8(60):34625-34633. doi: 10.1039/c8ra06589f. eCollection 2018 Oct 4. RSC Adv. 2018. PMID: 35548629 Free PMC article.
-
Development of a dual-wavelength fluorescent nanoprobe for in vivo and in vitro cell tracking consecutively.Bioorg Med Chem. 2019 May 1;27(9):1855-1862. doi: 10.1016/j.bmc.2019.03.036. Epub 2019 Mar 19. Bioorg Med Chem. 2019. PMID: 30910476 Free PMC article.
-
Extracellular matrix mediators of metastatic cell colonization characterized using scaffold mimics of the pre-metastatic niche.Acta Biomater. 2016 Mar;33:13-24. doi: 10.1016/j.actbio.2016.01.043. Epub 2016 Feb 1. Acta Biomater. 2016. PMID: 26844426 Free PMC article.
-
CXCL12 loaded-dermal filler captures CXCR4 expressing melanoma circulating tumor cells.Cell Death Dis. 2019 Jul 22;10(8):562. doi: 10.1038/s41419-019-1796-6. Cell Death Dis. 2019. PMID: 31332163 Free PMC article.
-
Microporous scaffolds loaded with immunomodulatory lentivirus to study the contribution of immune cell populations to tumor cell recruitment in vivo.Biotechnol Bioeng. 2020 Jan;117(1):210-222. doi: 10.1002/bit.27179. Epub 2019 Oct 3. Biotechnol Bioeng. 2020. PMID: 31544959 Free PMC article.
References
-
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. - PubMed
-
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. - PubMed
-
- Welch DR. Technical considerations for studying cancer metastasis in vivo. Clin Exp Metastasis. 1997;15:272–306. - PubMed
-
- Yamamoto M, Kikuchi H, Ohta M, Kawabata T, Hiramatsu Y, Kondo K, et al. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res. 2008;68:9754–9762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

