Analysis of the interaction between the essential herpes simplex virus 1 tegument proteins VP16 and VP1/2
- PMID: 22013045
- PMCID: PMC3255927
- DOI: 10.1128/JVI.05981-11
Analysis of the interaction between the essential herpes simplex virus 1 tegument proteins VP16 and VP1/2
Abstract
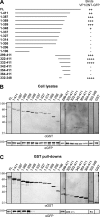
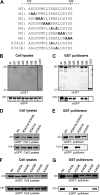
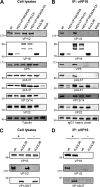
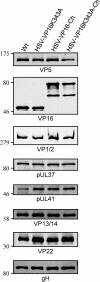
The incorporation of tegument proteins into the herpes simplex virus 1 (HSV-1) virion during virion assembly is thought to be a complex, multistage process occurring via numerous interactions between the tegument and the capsid, within the tegument, and between the tegument and the envelope. Here, we set out to examine if the direct interaction between two essential tegument proteins VP1/2 and VP16 is required for connecting the inner tegument with the outer tegument. By using glutathione S-transferase (GST) pulldowns, we identified an essential role of lysine 343 in VP16, mutation of which to a neutral amino acid abrogated the interaction between VP1/2 and VP16. When the K343A substitution was inserted into the gene encoding VP16 (UL48) of the viral genome, HSV-1 replicated successfully although its growth was delayed, and final titers were reduced compared to titers of wild-type virus. Surprisingly, the mutated VP16 was incorporated into virions at levels similar to those of wild-type VP16. However, the analysis of VP16 on cytoplasmic capsids by fluorescence microscopy showed that VP16 associated with cytoplasmic capsids less efficiently when the VP16-VP1/2 interaction was inhibited. This implies that the direct interaction between VP1/2 and VP16 is important for the efficiency/timing of viral assembly but is not essential for HSV-1 replication in cell culture. These data also support the notion that the incorporation of tegument proteins into the herpesviruses is a very complex process with significant redundancy.
Figures

Similar articles
-
Conserved Tryptophan Motifs in the Large Tegument Protein pUL36 Are Required for Efficient Secondary Envelopment of Herpes Simplex Virus Capsids.J Virol. 2016 May 12;90(11):5368-5383. doi: 10.1128/JVI.03167-15. Print 2016 Jun 1. J Virol. 2016. PMID: 27009950 Free PMC article.
-
Incorporation of the herpes simplex virus type 1 tegument protein VP22 into the virus particle is independent of interaction with VP16.Virology. 2007 Dec 20;369(2):263-80. doi: 10.1016/j.virol.2007.07.020. Epub 2007 Sep 20. Virology. 2007. PMID: 17888478
-
Quantitative Evaluation of Protein Heterogeneity within Herpes Simplex Virus 1 Particles.J Virol. 2017 Apr 28;91(10):e00320-17. doi: 10.1128/JVI.00320-17. Print 2017 May 15. J Virol. 2017. PMID: 28275191 Free PMC article.
-
[Research Advances in VP16 of the Herpes Virus].Bing Du Xue Bao. 2016 Nov;32(6):817-24. Bing Du Xue Bao. 2016. PMID: 30004657 Review. Chinese.
-
Early expression of herpes simplex virus (HSV) proteins and reactivation of latent infection.Folia Microbiol (Praha). 2000;45(1):7-28. doi: 10.1007/BF02817445. Folia Microbiol (Praha). 2000. PMID: 11200675 Review.
Cited by
-
Differential protein partitioning within the herpesvirus tegument and envelope underlies a complex and variable virion architecture.Proc Natl Acad Sci U S A. 2013 Apr 23;110(17):E1613-20. doi: 10.1073/pnas.1221896110. Epub 2013 Apr 8. Proc Natl Acad Sci U S A. 2013. PMID: 23569236 Free PMC article.
-
Dual Function of the pUL7-pUL51 Tegument Protein Complex in Herpes Simplex Virus 1 Infection.J Virol. 2017 Jan 3;91(2):e02196-16. doi: 10.1128/JVI.02196-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27852850 Free PMC article.
-
The precise function of alphaherpesvirus tegument proteins and their interactions during the viral life cycle.Front Microbiol. 2024 Jul 2;15:1431672. doi: 10.3389/fmicb.2024.1431672. eCollection 2024. Front Microbiol. 2024. PMID: 39015737 Free PMC article. Review.
-
Structural analysis of herpes simplex virus by optical super-resolution imaging.Nat Commun. 2015 Jan 22;6:5980. doi: 10.1038/ncomms6980. Nat Commun. 2015. PMID: 25609143 Free PMC article.
-
Insights into herpesvirus tegument organization from structural analyses of the 970 central residues of HSV-1 UL36 protein.J Biol Chem. 2015 Apr 3;290(14):8820-33. doi: 10.1074/jbc.M114.612838. Epub 2015 Feb 12. J Biol Chem. 2015. PMID: 25678705 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

