Na Channel β Subunits: Overachievers of the Ion Channel Family
- PMID: 22007171
- PMCID: PMC3181431
- DOI: 10.3389/fphar.2011.00053
Na Channel β Subunits: Overachievers of the Ion Channel Family
Abstract
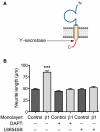
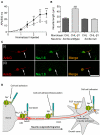
Voltage-gated Na(+) channels (VGSCs) in mammals contain a pore-forming α subunit and one or more β subunits. There are five mammalian β subunits in total: β1, β1B, β2, β3, and β4, encoded by four genes: SCN1B-SCN4B. With the exception of the SCN1B splice variant, β1B, the β subunits are type I topology transmembrane proteins. In contrast, β1B lacks a transmembrane domain and is a secreted protein. A growing body of work shows that VGSC β subunits are multifunctional. While they do not form the ion channel pore, β subunits alter gating, voltage-dependence, and kinetics of VGSCα subunits and thus regulate cellular excitability in vivo. In addition to their roles in channel modulation, β subunits are members of the immunoglobulin superfamily of cell adhesion molecules and regulate cell adhesion and migration. β subunits are also substrates for sequential proteolytic cleavage by secretases. An example of the multifunctional nature of β subunits is β1, encoded by SCN1B, that plays a critical role in neuronal migration and pathfinding during brain development, and whose function is dependent on Na(+) current and γ-secretase activity. Functional deletion of SCN1B results in Dravet Syndrome, a severe and intractable pediatric epileptic encephalopathy. β subunits are emerging as key players in a wide variety of physiopathologies, including epilepsy, cardiac arrhythmia, multiple sclerosis, Huntington's disease, neuropsychiatric disorders, neuropathic and inflammatory pain, and cancer. β subunits mediate multiple signaling pathways on different timescales, regulating electrical excitability, adhesion, migration, pathfinding, and transcription. Importantly, some β subunit functions may operate independently of α subunits. Thus, β subunits perform critical roles during development and disease. As such, they may prove useful in disease diagnosis and therapy.
Keywords: adhesion; development; excitability; voltage-gated Na+ channel; β subunit.
Figures
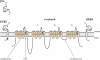

Similar articles
-
Voltage-gated Na+ channels: potential for beta subunits as therapeutic targets.Expert Opin Ther Targets. 2008 Sep;12(9):1191-203. doi: 10.1517/14728222.12.9.1191. Expert Opin Ther Targets. 2008. PMID: 18694383 Free PMC article.
-
An emerging role for voltage-gated Na+ channels in cellular migration: regulation of central nervous system development and potentiation of invasive cancers.Neuroscientist. 2008 Dec;14(6):571-83. doi: 10.1177/1073858408320293. Epub 2008 Oct 20. Neuroscientist. 2008. PMID: 18940784 Free PMC article. Review.
-
Developmental and Regulatory Functions of Na(+) Channel Non-pore-forming β Subunits.Curr Top Membr. 2016;78:315-51. doi: 10.1016/bs.ctm.2016.07.003. Epub 2016 Aug 2. Curr Top Membr. 2016. PMID: 27586289 Review.
-
The role of non-pore-forming β subunits in physiology and pathophysiology of voltage-gated sodium channels.Handb Exp Pharmacol. 2014;221:51-89. doi: 10.1007/978-3-642-41588-3_4. Handb Exp Pharmacol. 2014. PMID: 24737232 Review.
-
β1-C121W Is Down But Not Out: Epilepsy-Associated Scn1b-C121W Results in a Deleterious Gain-of-Function.J Neurosci. 2016 Jun 8;36(23):6213-24. doi: 10.1523/JNEUROSCI.0405-16.2016. J Neurosci. 2016. PMID: 27277800 Free PMC article.
Cited by
-
A uniquely adaptable pore is consistent with NALCN being an ion sensor.Channels (Austin). 2013 Mar-Apr;7(2):60-8. doi: 10.4161/chan.23981. Epub 2013 Feb 26. Channels (Austin). 2013. PMID: 23442378 Free PMC article.
-
Therapeutic Potential of Targeting Regulated Intramembrane Proteolysis Mechanisms of Voltage-Gated Ion Channel Subunits and Cell Adhesion Molecules.Pharmacol Rev. 2022 Oct;74(4):1028-1048. doi: 10.1124/pharmrev.121.000340. Pharmacol Rev. 2022. PMID: 36113879 Free PMC article. Review.
-
Relative contribution of TARPs γ-2 and γ-7 to cerebellar excitatory synaptic transmission and motor behavior.Proc Natl Acad Sci U S A. 2015 Jan 27;112(4):E371-9. doi: 10.1073/pnas.1423670112. Epub 2015 Jan 12. Proc Natl Acad Sci U S A. 2015. PMID: 25583485 Free PMC article.
-
Multilayer control of cardiac electrophysiology by microRNAs.J Mol Cell Cardiol. 2022 May;166:107-115. doi: 10.1016/j.yjmcc.2022.02.007. Epub 2022 Mar 3. J Mol Cell Cardiol. 2022. PMID: 35247375 Free PMC article. Review.
-
Voltage-dependent activation of Rac1 by Nav 1.5 channels promotes cell migration.J Cell Physiol. 2020 Apr;235(4):3950-3972. doi: 10.1002/jcp.29290. Epub 2019 Oct 15. J Cell Physiol. 2020. PMID: 31612502 Free PMC article.
References
-
- Andrikopoulos P., Fraser S. P., Patterson L., Ahmad Z., Burcu H., Ottaviani D., Diss J. K., Box C., Eccles S. A., Djamgoz M. B. (2011). Angiogenic functions of voltage-gated Na+ channels in human endothelial cells: modulation of vascular endothelial growth factor (VEGF) signaling. J. Biol. Chem. 286, 16846–1686010.1074/jbc.M110.187559 - DOI - PMC - PubMed
-
- Aronica E., Troost D., Rozemuller A. J., Yankaya B., Jansen G. H., Isom L. L., Gorter J. A. (2003). Expression and regulation of voltage-gated sodium channel beta1 subunit protein in human gliosis-associated pathologies. Acta Neuropathol. 105, 515–523 - PubMed
-
- Audenaert D., Claes L., Ceulemans B., Lofgren A., Van Broeckhoven C., De Jonghe P. (2003). A deletion in SCN1B is associated with febrile seizures and early-onset absence epilepsy. Neurology 61, 854–856 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

