The efficacy of an anti-CD4 monoclonal antibody for HIV-1 treatment
- PMID: 22001594
- PMCID: PMC4388049
- DOI: 10.1016/j.antiviral.2011.09.010
The efficacy of an anti-CD4 monoclonal antibody for HIV-1 treatment
Abstract
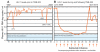
The availability of 24 antiretroviral (ARV) drugs within six distinct drug classes has transformed HIV-1 infection (AIDS) into a treatable chronic disease. However, the ability of HIV-1 to develop resistance to multiple classes continues to present challenges to the treatment of many ARV treatment-experienced patients. In this case report, we describe the response to ibalizumab, an investigational CD4-binding monoclonal antibody (mAb), in a patient with advanced immunodeficiency and high-level five-class antiretroviral resistance. After starting an ibalizumab-based salvage regimen, the patient had an approximately 4.0 log(10) reduction in viral load. An inadvertently missed infusion at week 32 led to the rapid loss of virologic response and decreased susceptibility to the remainder of the patient's salvage therapy regimen. Following the reinstitution of ibalizumab, phenotypic and genotypic resistance to ibalizumab was detected. Nonetheless, plasma HIV-1 RNA levels stabilized at ∼2.0 log(10) copies/ml below pre-ibalizumab levels. Continued ARV drug development may yield additional clinical and public health benefits. This report illustrates the promise of mAbs for HIV-1 therapy in highly treatment-experienced patients. Therapeutic mAbs may also have a role in pre-exposure prophylaxis in high-risk uninfected populations and may facilitate directly observed therapy (DOT) if two or more synergistic long acting agents become available.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Phase 3 Study of Ibalizumab for Multidrug-Resistant HIV-1.N Engl J Med. 2018 Aug 16;379(7):645-654. doi: 10.1056/NEJMoa1711460. N Engl J Med. 2018. PMID: 30110589 Clinical Trial.
-
Ibalizumab and Fostemsavir in the Management of Heavily Pre-Treated HIV-infected Patients.Recent Pat Antiinfect Drug Discov. 2018;13(3):190-197. doi: 10.2174/1574891X13666181031120019. Recent Pat Antiinfect Drug Discov. 2018. PMID: 30378502 Review.
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
-
Loss of asparagine-linked glycosylation sites in variable region 5 of human immunodeficiency virus type 1 envelope is associated with resistance to CD4 antibody ibalizumab.J Virol. 2011 Apr;85(8):3872-80. doi: 10.1128/JVI.02237-10. Epub 2011 Feb 2. J Virol. 2011. PMID: 21289125 Free PMC article.
-
Baseline CD4(+) T-cell counts and weighted background susceptibility scores strongly predict response to maraviroc regimens in treatment-experienced patients.Antivir Ther. 2011;16(3):395-404. doi: 10.3851/IMP1759. Antivir Ther. 2011. PMID: 21555822
Cited by
-
Mapping Determinants of Virus Neutralization and Viral Escape for Rational Design of a Hepatitis C Virus Vaccine.Front Immunol. 2018 May 31;9:1194. doi: 10.3389/fimmu.2018.01194. eCollection 2018. Front Immunol. 2018. PMID: 29904384 Free PMC article. Review.
-
New Mechanism for Release of Endosomal Contents: Osmotic Lysis via Nigericin-Mediated K+/H+ Exchange.Bioconjug Chem. 2018 Apr 18;29(4):1047-1059. doi: 10.1021/acs.bioconjchem.7b00714. Epub 2018 Mar 2. Bioconjug Chem. 2018. PMID: 29446616 Free PMC article.
-
Combinatorial anti-HIV gene therapy: using a multipronged approach to reach beyond HAART.Gene Ther. 2013 Jul;20(7):695-702. doi: 10.1038/gt.2012.98. Epub 2013 Jan 31. Gene Ther. 2013. PMID: 23364313 Free PMC article. Review.
-
Potent suppression of HIV-1 cell attachment by Kudzu root extract.Retrovirology. 2018 Sep 20;15(1):64. doi: 10.1186/s12977-018-0446-x. Retrovirology. 2018. PMID: 30236131 Free PMC article.
-
Ibalizumab Targeting CD4 Receptors, An Emerging Molecule in HIV Therapy.Front Microbiol. 2017 Nov 27;8:2323. doi: 10.3389/fmicb.2017.02323. eCollection 2017. Front Microbiol. 2017. PMID: 29230203 Free PMC article. Review.
References
-
- Boon L, Holland B, Gordon W, Liu P, Shiau F, Shanahan W, Reimann KA, Fung M. Development of anti-CD4 MAb hu5A8 for treatment of HIV-1 infection: preclinical assessment in non-human primates. Toxicology. 2002;172:191–203. - PubMed
-
- Burkly LC, Olson D, Shapiro R, Winkler G, Rosa JJ, Thomas DW, Williams C, Chisholm P. Inhibition of HIV infection by a novel CD4 domain 2-specific monoclonal antibody. Dissecting the basis for its inhibitory effect on HIV-induced cell fusion. J. Immunol. 1992;149:1779–1787. - PubMed
-
- Huber M, Olson WC, Trkola A. Antibodies for HIV treatment and prevention: window of opportunity? Curr. Top. Microbiol. Immunol. 2008;317:39–66. - PubMed
-
- Jacobson JM, Kuritzkes DR, Godofsky E, DeJesus E, Larson JA, Weinheimer SP, Lewis ST. Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob. Agents Chemother. 2009;53:450–457. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

