Ubiquitin carboxyl-terminal hydrolase 1 (UCHL1) is a potential tumour suppressor in prostate cancer and is frequently silenced by promoter methylation
- PMID: 21999842
- PMCID: PMC3212821
- DOI: 10.1186/1476-4598-10-129
Ubiquitin carboxyl-terminal hydrolase 1 (UCHL1) is a potential tumour suppressor in prostate cancer and is frequently silenced by promoter methylation
Abstract
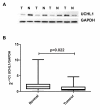
Background: We have previously reported significant downregulation of ubiquitin carboxyl-terminal hydrolase 1 (UCHL1) in prostate cancer (PCa) compared to the surrounding benign tissue. UCHL1 plays an important role in ubiquitin system and different cellular processes such as cell proliferation and differentiation. We now show that the underlying mechanism of UCHL1 downregulation in PCa is linked to its promoter hypermethylation. Furthermore, we present evidences that UCHL1 expression can affect the behavior of prostate cancer cells in different ways.
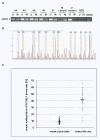
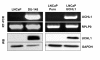
Results: Methylation specific PCR analysis results showed a highly methylated promoter region for UCHL1 in 90% (18/20) of tumor tissue compared to 15% (3/20) of normal tissues from PCa patients. Pyrosequencing results confirmed a mean methylation of 41.4% in PCa whereas only 8.6% in normal tissues. To conduct functional analysis of UCHL1 in PCa, UCHL1 is overexpressed in LNCaP cells whose UCHL1 expression is normally suppressed by promoter methylation and found that UCHL1 has the ability to decrease the rate of cell proliferation and suppresses anchorage-independent growth of these cells. In further analysis, we found evidence that exogenous expression of UCHL1 suppress LNCaP cells growth probably via p53-mediated inhibition of Akt/PKB phosphorylation and also via accumulation of p27kip1 a cyclin dependant kinase inhibitor of cell cycle regulating proteins. Notably, we also observed that exogenous expression of UCHL1 induced a senescent phenotype that was detected by using the SA-ß-gal assay and might be due to increased p14ARF, p53, p27kip1 and decreased MDM2.
Conclusion: From these results, we propose that UCHL1 downregulation via promoter hypermethylation plays an important role in various molecular aspects of PCa biology, such as morphological diversification and regulation of proliferation.
Figures

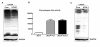


Similar articles
-
Epigenetic identification of ubiquitin carboxyl-terminal hydrolase L1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors.Hepatology. 2008 Aug;48(2):508-18. doi: 10.1002/hep.22343. Hepatology. 2008. PMID: 18666234
-
The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma.Clin Cancer Res. 2010 Jun 1;16(11):2949-58. doi: 10.1158/1078-0432.CCR-09-3178. Epub 2010 Apr 15. Clin Cancer Res. 2010. PMID: 20395212
-
The ubiquitin peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer.PLoS One. 2012;7(1):e29783. doi: 10.1371/journal.pone.0029783. Epub 2012 Jan 18. PLoS One. 2012. PMID: 22279545 Free PMC article.
-
Gene methylation in gastric cancer.Clin Chim Acta. 2013 Sep 23;424:53-65. doi: 10.1016/j.cca.2013.05.002. Epub 2013 May 10. Clin Chim Acta. 2013. PMID: 23669186 Review.
-
Ubiquitin carboxy-terminal hydrolase L1 - physiology and pathology.Cell Biochem Funct. 2020 Jul;38(5):533-540. doi: 10.1002/cbf.3527. Epub 2020 Mar 24. Cell Biochem Funct. 2020. PMID: 32207552 Review.
Cited by
-
The deubiquitinating enzyme UCHL1 is a favorable prognostic marker in neuroblastoma as it promotes neuronal differentiation.J Exp Clin Cancer Res. 2018 Oct 25;37(1):258. doi: 10.1186/s13046-018-0931-z. J Exp Clin Cancer Res. 2018. PMID: 30359286 Free PMC article.
-
Novel protein and immune response markers of human serous tubal intraepithelial carcinoma of the ovary.Cancer Biomark. 2019;26(4):471-479. doi: 10.3233/CBM-190528. Cancer Biomark. 2019. PMID: 31658047 Free PMC article.
-
Implications of Long Non-Coding RNAs in Age-Altered Proteostasis.Aging Dis. 2020 May 9;11(3):692-704. doi: 10.14336/AD.2019.0814. eCollection 2020 May. Aging Dis. 2020. PMID: 32489713 Free PMC article. Review.
-
Establishment of an ovarian cancer omentum metastasis-related prognostic model by integrated analysis of scRNA-seq and bulk RNA-seq.J Ovarian Res. 2022 Nov 23;15(1):123. doi: 10.1186/s13048-022-01059-0. J Ovarian Res. 2022. PMID: 36424614 Free PMC article.
-
Ubiquitin C-terminal hydrolase-L1 has prognostic relevance and is a therapeutic target for high-grade neuroendocrine lung cancers.Cancer Sci. 2020 Feb;111(2):610-620. doi: 10.1111/cas.14284. Epub 2020 Jan 19. Cancer Sci. 2020. PMID: 31845438 Free PMC article.
References
-
- Ummanni R, Mundt F, Pospisil H, Venz S, Scharf C, Barett C, Falth M, Kollermann J, Walther R, Schlomm T. et al.Identification of Clinically Relevant Protein Targets in Prostate Cancer with 2D-DIGE Coupled Mass Spectrometry and Systems Biology Network Platform. PLoS One. 2011;6:e16833. doi: 10.1371/journal.pone.0016833. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

