Mechanisms of Candida albicans trafficking to the brain
- PMID: 21998592
- PMCID: PMC3188548
- DOI: 10.1371/journal.ppat.1002305
Mechanisms of Candida albicans trafficking to the brain
Abstract
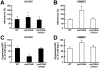
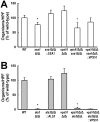
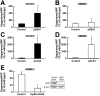
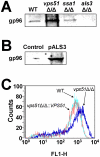
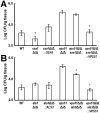
During hematogenously disseminated disease, Candida albicans infects most organs, including the brain. We discovered that a C. albicans vps51Δ/Δ mutant had significantly increased tropism for the brain in the mouse model of disseminated disease. To investigate the mechanisms of this enhanced trafficking to the brain, we studied the interactions of wild-type C. albicans and the vps51Δ/Δ mutant with brain microvascular endothelial cells in vitro. These studies revealed that C. albicans invasion of brain endothelial cells is mediated by the fungal invasins, Als3 and Ssa1. Als3 binds to the gp96 heat shock protein, which is expressed on the surface of brain endothelial cells, but not human umbilical vein endothelial cells, whereas Ssa1 binds to a brain endothelial cell receptor other than gp96. The vps51Δ/Δ mutant has increased surface expression of Als3, which is a major cause of the increased capacity of this mutant to both invade brain endothelial cells in vitro and traffic to the brain in mice. Therefore, during disseminated disease, C. albicans traffics to and infects the brain by binding to gp96, a unique receptor that is expressed specifically on the surface of brain endothelial cells.
Conflict of interest statement
SGF is one of the founders of NovaDigm Therapeutics and serves on its scientific advisory board.
Figures

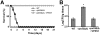

Similar articles
-
Investigation of the function of Candida albicans Als3 by heterologous expression in Candida glabrata.Infect Immun. 2013 Jul;81(7):2528-35. doi: 10.1128/IAI.00013-13. Epub 2013 Apr 29. Infect Immun. 2013. PMID: 23630968 Free PMC article.
-
SR-like RNA-binding protein Slr1 affects Candida albicans filamentation and virulence.Infect Immun. 2013 Apr;81(4):1267-76. doi: 10.1128/IAI.00864-12. Epub 2013 Feb 4. Infect Immun. 2013. PMID: 23381995 Free PMC article.
-
Role of retrograde trafficking in stress response, host cell interactions, and virulence of Candida albicans.Eukaryot Cell. 2014 Feb;13(2):279-87. doi: 10.1128/EC.00295-13. Epub 2013 Dec 20. Eukaryot Cell. 2014. PMID: 24363364 Free PMC article.
-
Candida albicans Als3, a multifunctional adhesin and invasin.Eukaryot Cell. 2011 Feb;10(2):168-73. doi: 10.1128/EC.00279-10. Epub 2010 Nov 29. Eukaryot Cell. 2011. PMID: 21115738 Free PMC article. Review.
-
Fungal invasion of epithelial cells.Microbiol Res. 2014 Nov;169(11):803-10. doi: 10.1016/j.micres.2014.02.013. Epub 2014 Mar 12. Microbiol Res. 2014. PMID: 24670964 Review.
Cited by
-
Pathogens infecting the central nervous system.PLoS Pathog. 2022 Feb 24;18(2):e1010234. doi: 10.1371/journal.ppat.1010234. eCollection 2022 Feb. PLoS Pathog. 2022. PMID: 35202445 Free PMC article. No abstract available.
-
New signaling pathways govern the host response to C. albicans infection in various niches.Genome Res. 2015 May;25(5):679-89. doi: 10.1101/gr.187427.114. Epub 2015 Apr 9. Genome Res. 2015. PMID: 25858952 Free PMC article.
-
Can host receptors for fungi be targeted for treatment of fungal infections?Trends Microbiol. 2013 Aug;21(8):389-96. doi: 10.1016/j.tim.2013.05.006. Epub 2013 Jun 21. Trends Microbiol. 2013. PMID: 23796589 Free PMC article. Review.
-
Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections.J Fungi (Basel). 2020 Oct 22;6(4):239. doi: 10.3390/jof6040239. J Fungi (Basel). 2020. PMID: 33105672 Free PMC article. Review.
-
Role of endothelial cell septin 7 in the endocytosis of Candida albicans.mBio. 2013 Dec 17;4(6):e00542-13. doi: 10.1128/mBio.00542-13. mBio. 2013. PMID: 24345743 Free PMC article.
References
-
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. - PubMed
-
- Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48:1695–1703. - PubMed
-
- Parker JC, Jr, McCloskey JJ, Lee RS. Human cerebral candidosis--a postmortem evaluation of 19 patients. Hum Pathol. 1981;12:23–28. - PubMed
-
- Faix RG, Chapman RL. Central nervous system candidiasis in the high-risk neonate. Semin Perinatol. 2003;27:384–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

