Successful transmission of a retrovirus depends on the commensal microbiota
- PMID: 21998394
- PMCID: PMC3519937
- DOI: 10.1126/science.1210718
Successful transmission of a retrovirus depends on the commensal microbiota
Abstract
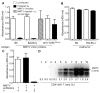
To establish chronic infections, viruses must develop strategies to evade the host's immune responses. Many retroviruses, including mouse mammary tumor virus (MMTV), are transmitted most efficiently through mucosal surfaces rich in microbiota. We found that MMTV, when ingested by newborn mice, stimulates a state of unresponsiveness toward viral antigens. This process required the intestinal microbiota, as antibiotic-treated mice or germ-free mice did not transmit infectious virus to their offspring. MMTV-bound bacterial lipopolysaccharide triggered Toll-like receptor 4 and subsequent interleukin-6 (IL-6)-dependent induction of the inhibitory cytokine IL-10. Thus, MMTV has evolved to rely on the interaction with the microbiota to induce an immune evasion pathway. Together, these findings reveal the fundamental importance of commensal microbiota in viral infections.
Figures

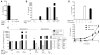
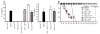
Comment in
-
Microbiology. Gut bacteria lend a molecular hand to viruses.Science. 2011 Oct 14;334(6053):168. doi: 10.1126/science.334.6053.168. Science. 2011. PMID: 21998362 No abstract available.
-
Viral infection. The gut microbiota: friend or foe?Nat Rev Microbiol. 2011 Nov 2;9(12):831. doi: 10.1038/nrmicro2691. Nat Rev Microbiol. 2011. PMID: 22048739
Similar articles
-
Microbiology. Gut bacteria lend a molecular hand to viruses.Science. 2011 Oct 14;334(6053):168. doi: 10.1126/science.334.6053.168. Science. 2011. PMID: 21998362 No abstract available.
-
B and T cells are required for mouse mammary tumor virus spread within the mammary gland.J Immunol. 1998 Sep 1;161(5):2375-82. J Immunol. 1998. PMID: 9725233
-
Subversion of the innate immune system by a retrovirus.Nat Immunol. 2003 Jun;4(6):573-8. doi: 10.1038/ni926. Epub 2003 May 5. Nat Immunol. 2003. PMID: 12730691
-
Mouse mammary tumor virus and the immune system.Immunol Res. 2003;27(2-3):469-80. doi: 10.1385/IR:27:2-3:469. Immunol Res. 2003. PMID: 12857990 Review.
-
Immune response to MMTV infection.Front Biosci. 2007 Jan 1;12:1594-609. doi: 10.2741/2172. Front Biosci. 2007. PMID: 17127406 Review.
Cited by
-
The microbiome and innate immunity.Nature. 2016 Jul 7;535(7610):65-74. doi: 10.1038/nature18847. Nature. 2016. PMID: 27383981 Review.
-
Microbiota and Its Role on Viral Evasion: Is It With Us or Against Us?Front Cell Infect Microbiol. 2019 Jul 18;9:256. doi: 10.3389/fcimb.2019.00256. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31380299 Free PMC article. Review.
-
Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus.Cell Host Microbe. 2014 Jan 15;15(1):36-46. doi: 10.1016/j.chom.2013.12.004. Cell Host Microbe. 2014. PMID: 24439896 Free PMC article.
-
An Update on the Mutual Impact between SARS-CoV-2 Infection and Gut Microbiota.Viruses. 2022 Aug 15;14(8):1774. doi: 10.3390/v14081774. Viruses. 2022. PMID: 36016396 Free PMC article. Review.
-
Autophagy, viruses, and intestinal immunity.Curr Opin Gastroenterol. 2014 Nov;30(6):539-46. doi: 10.1097/MOG.0000000000000121. Curr Opin Gastroenterol. 2014. PMID: 25291356 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- AI090084/AI/NIAID NIH HHS/United States
- P30 DK042086/DK/NIDDK NIH HHS/United States
- R01 AI090084/AI/NIAID NIH HHS/United States
- P30 CA014599/CA/NCI NIH HHS/United States
- DK42086/DK/NIDDK NIH HHS/United States
- T32 AI065382-01/AI/NIAID NIH HHS/United States
- R01 AI082418/AI/NIAID NIH HHS/United States
- AI082418/AI/NIAID NIH HHS/United States
- R01 CA134667/CA/NCI NIH HHS/United States
- R56 AI090084/AI/NIAID NIH HHS/United States
- T32GM007183/GM/NIGMS NIH HHS/United States
- CA100383/CA/NCI NIH HHS/United States
- R01 CA100383/CA/NCI NIH HHS/United States
- T32 GM007183/GM/NIGMS NIH HHS/United States
- T32 AI065382/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

