Protective and pathogenic functions of macrophage subsets
- PMID: 21997792
- PMCID: PMC3422549
- DOI: 10.1038/nri3073
Protective and pathogenic functions of macrophage subsets
Abstract
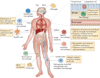
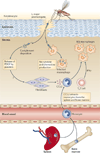
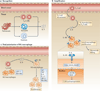
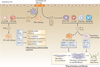
Macrophages are strategically located throughout the body tissues, where they ingest and process foreign materials, dead cells and debris and recruit additional macrophages in response to inflammatory signals. They are highly heterogeneous cells that can rapidly change their function in response to local microenvironmental signals. In this Review, we discuss the four stages of orderly inflammation mediated by macrophages: recruitment to tissues; differentiation and activation in situ; conversion to suppressive cells; and restoration of tissue homeostasis. We also discuss the protective and pathogenic functions of the various macrophage subsets in antimicrobial defence, antitumour immune responses, metabolism and obesity, allergy and asthma, tumorigenesis, autoimmunity, atherosclerosis, fibrosis and wound healing. Finally, we briefly discuss the characterization of macrophage heterogeneity in humans.
Figures
Similar articles
-
Inflammatory response of macrophages in infection.Hepatobiliary Pancreat Dis Int. 2014 Apr;13(2):138-52. doi: 10.1016/s1499-3872(14)60024-2. Hepatobiliary Pancreat Dis Int. 2014. PMID: 24686541 Review.
-
Macrophages in spinal cord injury: phenotypic and functional change from exposure to myelin debris.Glia. 2015 Apr;63(4):635-51. doi: 10.1002/glia.22774. Epub 2014 Nov 28. Glia. 2015. PMID: 25452166 Free PMC article.
-
Macrophage-mediated inflammation in metabolic disease.Nat Rev Immunol. 2011 Oct 10;11(11):738-49. doi: 10.1038/nri3071. Nat Rev Immunol. 2011. PMID: 21984069 Free PMC article. Review.
-
Macrophages in wound healing: activation and plasticity.Immunol Cell Biol. 2019 Mar;97(3):258-267. doi: 10.1111/imcb.12236. Epub 2019 Feb 11. Immunol Cell Biol. 2019. PMID: 30746824 Free PMC article. Review.
-
Tissue-resident macrophages.Nat Immunol. 2013 Oct;14(10):986-95. doi: 10.1038/ni.2705. Epub 2013 Sep 18. Nat Immunol. 2013. PMID: 24048120 Free PMC article. Review.
Cited by
-
Interleukin-4-induced β-catenin regulates the conversion of macrophages to multinucleated giant cells.Mol Immunol. 2013 Jun;54(2):157-63. doi: 10.1016/j.molimm.2012.12.004. Epub 2012 Dec 31. Mol Immunol. 2013. PMID: 23287596 Free PMC article.
-
Location, location, location: tissue-specific regulation of immune responses.J Leukoc Biol. 2013 Sep;94(3):409-21. doi: 10.1189/jlb.0413207. Epub 2013 Jul 3. J Leukoc Biol. 2013. PMID: 23825388 Free PMC article. Review.
-
Sisters in arms: myeloid and tubular epithelial cells shape renal innate immunity.Am J Physiol Renal Physiol. 2013 May 15;304(10):F1243-51. doi: 10.1152/ajprenal.00101.2013. Epub 2013 Mar 20. Am J Physiol Renal Physiol. 2013. PMID: 23515715 Free PMC article. Review.
-
CD4-Positive T Cells and M2 Macrophages Dominate the Peritoneal Infiltrate of Patients with Encapsulating Peritoneal Sclerosis.PLoS One. 2015 Apr 24;10(4):e0120174. doi: 10.1371/journal.pone.0120174. eCollection 2015. PLoS One. 2015. PMID: 25910222 Free PMC article.
-
Oncometabolism: A Paradigm for the Metabolic Remodeling of the Failing Heart.Int J Mol Sci. 2022 Nov 11;23(22):13902. doi: 10.3390/ijms232213902. Int J Mol Sci. 2022. PMID: 36430377 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

