Inhibition of the type I interferon antiviral response during arenavirus infection
- PMID: 21994626
- PMCID: PMC3185579
- DOI: 10.3390/v2112443
Inhibition of the type I interferon antiviral response during arenavirus infection
Abstract
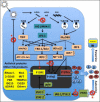
Arenaviruses merit interest both as tractable experimental model systems to study acute and persistent viral infections, and as clinically-important human pathogens. Several arenaviruses cause hemorrhagic fever (HF) disease in humans. In addition, evidence indicates that the globally-distributed prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) is a human pathogen of clinical significance in congenital infections, and also poses a great danger to immunosuppressed individuals. Arenavirus persistence and pathogenesis are facilitated by their ability to overcome the host innate immune response. Mammalian hosts have developed both membrane toll-like receptors (TLR) and cytoplasmic pattern recognition receptors (PRRs) that recognize specific pathogen-associated molecular patterns (PAMPs), resulting in activation of the transcription factors IRF3 or IRF7, or both, which together with NF-κB and ATF-2/c-JUN induce production of type I interferon (IFN-I). IFN-I plays a key role in host anti-microbial defense by mediating direct antiviral effects via up-regulation of IFN-I stimulated genes (ISGs), activating dendritic cells (DCs) and natural killer (NK) cells, and promoting the induction of adaptive responses. Accordingly, viruses have developed a plethora of strategies to disrupt the IFN-I mediated antiviral defenses of the host, and the viral gene products responsible for these disruptions are often major virulence determinants. IRF3- and IRF7-dependent induction of host innate immune responses is frequently targeted by viruses. Thus, the arenavirus nucleoprotein (NP) was shown to inhibit the IFN-I response by interfering with the activation of IRF3. This NP anti-IFN activity, together with alterations in the number and function of DCs observed in mice chronically infected with LCMV, likely play an important role in LCMV persistence in its murine host. In this review we will discuss current knowledge about the cellular and molecular mechanisms by which arenaviruses can subvert the host innate immune response and their implications for understanding HF arenaviral disease as well as arenavirus persistence in their natural hosts.
Keywords: arenavirus; dendritic cells; hemorrhagic fever; innate immunity; lymphocytic choriomeningitis virus; nucleoprotein; type I interferon.
Figures


Similar articles
-
Arenavirus nucleoprotein targets interferon regulatory factor-activating kinase IKKε.J Virol. 2012 Aug;86(15):7728-38. doi: 10.1128/JVI.00187-12. Epub 2012 Apr 24. J Virol. 2012. PMID: 22532683 Free PMC article.
-
Lymphocytic Choriomeningitis Virus Differentially Affects the Virus-Induced Type I Interferon Response and Mitochondrial Apoptosis Mediated by RIG-I/MAVS.J Virol. 2015 Jun;89(12):6240-50. doi: 10.1128/JVI.00610-15. Epub 2015 Apr 1. J Virol. 2015. PMID: 25833049 Free PMC article.
-
Arenavirus nucleoproteins prevent activation of nuclear factor kappa B.J Virol. 2012 Aug;86(15):8185-97. doi: 10.1128/JVI.07240-11. Epub 2012 May 23. J Virol. 2012. PMID: 22623788 Free PMC article.
-
Innate immune response to arenaviral infection: a focus on the highly pathogenic New World hemorrhagic arenaviruses.J Mol Biol. 2013 Dec 13;425(24):4893-903. doi: 10.1016/j.jmb.2013.09.028. Epub 2013 Sep 26. J Mol Biol. 2013. PMID: 24075870 Free PMC article. Review.
-
Novel antiviral strategies to combat human Arenavirus infections.Curr Mol Med. 2005 Dec;5(8):735-51. doi: 10.2174/156652405774962353. Curr Mol Med. 2005. PMID: 16375709 Review.
Cited by
-
Self-association of lymphocytic choriomeningitis virus nucleoprotein is mediated by its N-terminal region and is not required for its anti-interferon function.J Virol. 2012 Mar;86(6):3307-17. doi: 10.1128/JVI.05503-11. Epub 2012 Jan 18. J Virol. 2012. PMID: 22258244 Free PMC article.
-
Arenavirus nucleoprotein targets interferon regulatory factor-activating kinase IKKε.J Virol. 2012 Aug;86(15):7728-38. doi: 10.1128/JVI.00187-12. Epub 2012 Apr 24. J Virol. 2012. PMID: 22532683 Free PMC article.
-
Rapid differentiation of hiPSCs into functional oligodendrocytes using an OLIG2 synthetic modified messenger RNA.Commun Biol. 2022 Oct 14;5(1):1095. doi: 10.1038/s42003-022-04043-y. Commun Biol. 2022. PMID: 36241911 Free PMC article.
-
The transcription factor NFAT5 limits infection-induced type I interferon responses.J Exp Med. 2020 Mar 2;217(3):jem.20190449. doi: 10.1084/jem.20190449. J Exp Med. 2020. PMID: 31816635 Free PMC article.
-
Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection.Cell Host Microbe. 2012 Jun 14;11(6):631-42. doi: 10.1016/j.chom.2012.05.003. Cell Host Microbe. 2012. PMID: 22704623 Free PMC article.
References
-
- Zinkernagel RM. On cross-priming of MHC class I-specific CTL: Rule or exception. Eur J Immunol. 2002;32:2385–2392. - PubMed
-
- Oldstone MB. Arenaviruses. I. The epidemiology molecular and cell biology of arenaviruses. Introduction. Curr Top Microbiol Immunol. 2002;262:V–XII. - PubMed
-
- Buchmeier MJ, de la Torre JC, Peters CJ. Fields Virology. 5th ed. Vol. 2. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. Arenaviridae: The viruses and their replication; pp. 1791–1827.
-
- McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. - PubMed
-
- Peters CJ, Khan AS. Hantavirus pulmonary syndrome: The new American hemorrhagic fever. Clin Infect Dis. 2002;34:1224–1231. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

