Maturation Pathways of Cross-Reactive HIV-1 Neutralizing Antibodies
- PMID: 21994570
- PMCID: PMC3185542
- DOI: 10.3390/v1030802
Maturation Pathways of Cross-Reactive HIV-1 Neutralizing Antibodies
Abstract
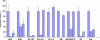
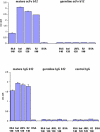
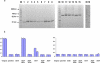
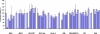
Several human monoclonal antibodies (hmAbs) and antibody fragments, including the best characterized in terms of structure-function b12 and Fab X5, exhibit relatively potent and broad HIV-1 neutralizing activity. However, the elicitation of b12 or b12-like antibodies in vivo by vaccine immunogens based on the HIV-1 envelope glycoprotein (Env) has not been successful. B12 is highly divergent from the closest corresponding germline antibody while X5 is less divergent. We have hypothesized that the relatively high degree of specific somatic hypermutations may preclude binding of the HIV-1 envelope glycoprotein (Env) to closest germline antibodies, and that identifying antibodies that are intermediates in the pathways to maturation could help design novel vaccine immunogens to guide the immune system for their enhanced elicitation. In support of this hypothesis we have previously found that a germline-like b12 (monovalent and bivalent scFv as an Fc fusion protein or IgG) lacks measurable binding to an Env as measured by ELISA with a sensitivity in the μM range [1]; here we present evidence confirming and expanding these findings for a panel of Envs. In contrast, a germline-like scFv X5 bound Env with high (nM) affinity. To begin to explore the maturation pathways of these antibodies we identified several possible b12 intermediate antibodies and tested their neutralizing activity. These intermediate antibodies neutralized only some HIV-1 isolates and with relatively weak potency. In contrast, germline-like scFv X5 neutralized a subset of the tested HIV-1 isolates with comparable efficiencies to that of the mature X5. These results could help explain the relatively high immunogenicity of the coreceptor binding site on gp120 and the abundance of CD4-induced (CD4i) antibodies in HIV-1-infected patients (X5 is a CD4i antibody) as well as the maturation pathway of X5. They also can help identify antigens that can bind specifically to b12 germline and intermediate antibodies that together with Envs could be used as a conceptually novel type of candidate vaccines. Such candidate vaccines based on two or more immunogens could help guiding the immune system through complex maturation pathways for elicitation of antibodies that are similar or identical to antibodies with known properties.
Keywords: HIV; antibody; escape; germline; immune responses; vaccine.
Figures



Similar articles
-
Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens.Biochem Biophys Res Commun. 2009 Dec 18;390(3):404-9. doi: 10.1016/j.bbrc.2009.09.029. Epub 2009 Sep 11. Biochem Biophys Res Commun. 2009. PMID: 19748484 Free PMC article.
-
Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes.Proc Natl Acad Sci U S A. 2002 May 14;99(10):6913-8. doi: 10.1073/pnas.102562599. Epub 2002 May 7. Proc Natl Acad Sci U S A. 2002. PMID: 11997472 Free PMC article.
-
Identification of Non-HIV Immunogens That Bind to Germline b12 Predecessors and Prime for Elicitation of Cross-clade Neutralizing HIV-1 Antibodies.PLoS One. 2015 May 26;10(5):e0126428. doi: 10.1371/journal.pone.0126428. eCollection 2015. PLoS One. 2015. PMID: 26010511 Free PMC article.
-
Novel approaches for identification of broadly cross-reactive HIV-1 neutralizing human monoclonal antibodies and improvement of their potency.Curr Pharm Des. 2007;13(2):203-12. doi: 10.2174/138161207779313669. Curr Pharm Des. 2007. PMID: 17269928 Review.
-
Designing immunogens to elicit broadly neutralizing antibodies to the HIV-1 envelope glycoprotein.Curr HIV Res. 2007 Nov;5(6):514-41. doi: 10.2174/157016207782418489. Curr HIV Res. 2007. PMID: 18045109 Review.
Cited by
-
The Relative Positioning of B and T Cell Epitopes Drives Immunodominance.Vaccines (Basel). 2022 Jul 31;10(8):1227. doi: 10.3390/vaccines10081227. Vaccines (Basel). 2022. PMID: 36016115 Free PMC article.
-
Heterologous prime-boost vaccination drives early maturation of HIV broadly neutralizing antibody precursors in humanized mice.Sci Transl Med. 2024 May 22;16(748):eadn0223. doi: 10.1126/scitranslmed.adn0223. Epub 2024 May 22. Sci Transl Med. 2024. PMID: 38753806 Free PMC article.
-
A Bivalent, Chimeric Rabies Virus Expressing Simian Immunodeficiency Virus Envelope Induces Multifunctional Antibody Responses.AIDS Res Hum Retroviruses. 2015 Nov;31(11):1126-38. doi: 10.1089/AID.2014.0319. Epub 2015 May 5. AIDS Res Hum Retroviruses. 2015. PMID: 25848984 Free PMC article.
-
Antibody mechanics on a membrane-bound HIV segment essential for GP41-targeted viral neutralization.Nat Struct Mol Biol. 2011 Oct 16;18(11):1235-43. doi: 10.1038/nsmb.2154. Nat Struct Mol Biol. 2011. PMID: 22002224 Free PMC article.
-
T Cell Subsets in the Germinal Center: Lessons from the Macaque Model.Front Immunol. 2018 Feb 26;9:348. doi: 10.3389/fimmu.2018.00348. eCollection 2018. Front Immunol. 2018. PMID: 29535724 Free PMC article. Review.
References
-
- Xiao X, Chen W, Feng Y, Zhu Z, Prabakaran P, Wang Y, Zhang MY, Longo NS, Dimitrov DS. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: Implications for evasion of immune responses and design of vaccine immunogens. Biochem Bioph Res Commun - PMC - PubMed
-
- Johnson WE, Desrosiers RC. Viral persistance: HIV's strategies of immune system evasion. Annu Rev Med. 2002;53:499–518. - PubMed
-
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. - PubMed
-
- Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–713. - PubMed
-
- Poignard P, Saphire EO, Parren PW, Burton DR. Gp120: Biologic aspects of structural features. Annu Rev Immunol. 2001;19:253–274. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

