The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string
- PMID: 2199063
- PMCID: PMC2753418
- DOI: 10.1016/0092-8674(90)90012-4
The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string
Abstract
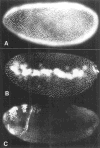
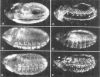
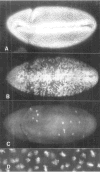
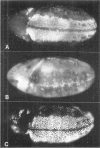
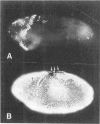
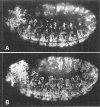
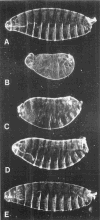
The string (stg) locus of Drosophila encodes a factor that is thought to trigger mitosis by activating the p34cdc2 protein kinase. stg is required for mitosis early in development and is transcribed in a dynamic pattern that anticipates the pattern of embryonic cell divisions. Here we show that differential cell cycle regulation during postblastoderm development (cell cycles 14-16) occurs in G2. We demonstrate that stg mRNA expressed from a heat shock promotor triggers mitosis, and an associated S phase, in G2 cells during these cycles. Hence, differential cell cycle timing at this developmental stage is controlled by stg. Finally, we use heat-induced stg expression to alter the normal pattern of embryonic mitoses. Surprisingly, the complex mitotic pattern evident during normal development is not essential for many features of pattern formation or for viability.
Figures
Similar articles
-
Drosophila development pulls the strings of the cell cycle.Bioessays. 1995 Jun;17(6):553-6. doi: 10.1002/bies.950170613. Bioessays. 1995. PMID: 7575498 Review.
-
Genetic control of cell division patterns in the Drosophila embryo.Cell. 1989 Apr 7;57(1):177-87. doi: 10.1016/0092-8674(89)90183-9. Cell. 1989. PMID: 2702688 Free PMC article.
-
string(cdc25) and cyclin E are required for patterned histone expression at different stages of Drosophila embryonic development.Dev Biol. 2004 Oct 1;274(1):82-93. doi: 10.1016/j.ydbio.2004.06.019. Dev Biol. 2004. PMID: 15355790
-
Activating the DNA damage checkpoint in a developmental context.Curr Biol. 2000 Feb 10;10(3):119-26. doi: 10.1016/s0960-9822(00)00300-6. Curr Biol. 2000. PMID: 10679321
-
Directing cell division during development.Science. 1989 Nov 3;246(4930):635-40. doi: 10.1126/science.2683080. Science. 1989. PMID: 2683080 Review.
Cited by
-
A map of directional genetic interactions in a metazoan cell.Elife. 2015 Mar 6;4:e05464. doi: 10.7554/eLife.05464. Elife. 2015. PMID: 25748138 Free PMC article.
-
The overlooked greatwall: a new perspective on mitotic control.Open Biol. 2012 Mar;2(3):120023. doi: 10.1098/rsob.120023. Open Biol. 2012. PMID: 22754657 Free PMC article. Review.
-
Zebrafish cdc25a is expressed during early development and limiting for post-blastoderm cell cycle progression.Dev Dyn. 2007 Dec;236(12):3427-35. doi: 10.1002/dvdy.21363. Dev Dyn. 2007. PMID: 17969147 Free PMC article.
-
SNR1 (INI1/SNF5) mediates important cell growth functions of the Drosophila Brahma (SWI/SNF) chromatin remodeling complex.Genetics. 2004 Sep;168(1):199-214. doi: 10.1534/genetics.104.029439. Genetics. 2004. PMID: 15454538 Free PMC article.
-
Rux is a cyclin-dependent kinase inhibitor (CKI) specific for mitotic cyclin-Cdk complexes.Curr Biol. 1999 Dec 2;9(23):1392-402. doi: 10.1016/s0960-9822(00)80084-6. Curr Biol. 1999. PMID: 10607563 Free PMC article.
References
-
- Bier E, Ackerman L, Barbel S, Jan L, Jan YN. Identification and characterization of a neuron specific antigen in Drosophila. Science. 1988;240:913–916. - PubMed
-
- Blow JJ, Laskey RA. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988;332:546–548. - PubMed
-
- Blumenthal AB, Kriegstein HJ, Hogness DS. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harbor Symp Quant Biol. 1974;38:205–223. - PubMed
-
- Bodmer R, Carretto R, Jan YN. Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron. 1989;3:21–32. - PubMed
-
- Ducommun B, Dreatta G, Young P, Beach D. Fission yeast cdc25 is a cell-cycle regulated protein. Biochem Biophys Res Commun. 1990;167:301–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

