Pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques
- PMID: 21986823
- PMCID: PMC3256015
- DOI: 10.1128/AAC.00597-11
Pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques
Abstract
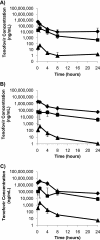
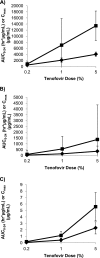
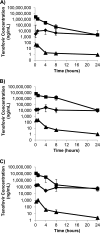
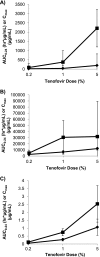
Tenofovir gel (1%) is being developed as a microbicide for the prevention of human immunodeficiency virus (HIV) infection and has been shown to reduce transmission to women by 39%. The gel also prevents infection in macaques when applied intravaginally or intrarectally prior to challenge with simian-human immunodeficiency virus (SHIV), but very little pharmacokinetic information for macaques is available to help extrapolate the data to humans and thus inform future development activities. We have determined the pharmacokinetics of tenofovir in macaques following intravaginal and intrarectal administration of 0.2, 1, and 5% gels. Plasma and vaginal and rectal fluid samples were collected up to 24 h after dosing, and at 24 h postdosing biopsy specimens were taken from the vaginal wall, cervix, and rectum. Following vaginal and rectal administration, tenofovir rapidly distributed to the matrices distal to the site of administration. In all matrices, exposure increased with increasing dose, and with the 1% and 5% formulations, concentrations remained detectable in most animals 24 h after dosing. At all doses, concentrations at the dosing site were typically 1 to 2 log units higher than those in the opposite compartment and 4 to 5 log units higher than those in plasma. Exposure in vaginal fluid after vaginal dosing was 58 to 82% lower than that in rectal fluid after rectal dosing, but plasma exposure was 1- to 2-fold greater after vaginal dosing than after rectal dosing. These data suggest that a tenofovir-based microbicide may have the potential to protect when exposure is via vaginal or anal intercourse, regardless of whether the microbicide is applied vaginally or rectally.
Figures
Similar articles
-
Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel.PLoS Med. 2008 Aug 5;5(8):e157; discussion e157. doi: 10.1371/journal.pmed.0050157. PLoS Med. 2008. PMID: 18684007 Free PMC article.
-
Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue.J Virol. 2012 Jan;86(2):718-25. doi: 10.1128/JVI.05842-11. Epub 2011 Nov 9. J Virol. 2012. PMID: 22072766 Free PMC article.
-
Efficacy of Vaginally Administered Gel Containing Emtricitabine and Tenofovir Against Repeated Rectal Simian Human Immunodeficiency Virus Exposures in Macaques.J Infect Dis. 2018 Sep 8;218(8):1284-1290. doi: 10.1093/infdis/jiy301. J Infect Dis. 2018. PMID: 29788316 Free PMC article.
-
Rectal microbicide development.Curr Opin HIV AIDS. 2012 Nov;7(6):526-33. doi: 10.1097/COH.0b013e3283582bc2. Curr Opin HIV AIDS. 2012. PMID: 23032732 Free PMC article. Review.
-
Systemic and topical use of monoclonal antibodies to prevent the sexual transmission of HIV.AIDS. 2017 Jul 17;31(11):1505-1517. doi: 10.1097/QAD.0000000000001521. AIDS. 2017. PMID: 28463876 Free PMC article. Review.
Cited by
-
Translational Models to Predict Target Concentrations for Pre-Exposure Prophylaxis in Women.AIDS Res Hum Retroviruses. 2022 Dec;38(12):909-923. doi: 10.1089/AID.2022.0057. Epub 2022 Oct 25. AIDS Res Hum Retroviruses. 2022. PMID: 36097755 Free PMC article. Review.
-
A Phase 1 Randomized, Open Label, Rectal Safety, Acceptability, Pharmacokinetic, and Pharmacodynamic Study of Three Formulations of Tenofovir 1% Gel (the CHARM-01 Study).PLoS One. 2015 May 5;10(5):e0125363. doi: 10.1371/journal.pone.0125363. eCollection 2015. PLoS One. 2015. PMID: 25942472 Free PMC article. Clinical Trial.
-
Phase I trial of pod-intravaginal rings delivering antiretroviral agents for HIV-1 prevention: Rectal drug exposure from vaginal dosing with tenofovir disoproxil fumarate, emtricitabine, and maraviroc.PLoS One. 2018 Aug 22;13(8):e0201952. doi: 10.1371/journal.pone.0201952. eCollection 2018. PLoS One. 2018. PMID: 30133534 Free PMC article. Clinical Trial.
-
Pharmacokinetics of a CCR5 inhibitor in rhesus macaques following vaginal, rectal and oral application.J Antimicrob Chemother. 2014 May;69(5):1325-9. doi: 10.1093/jac/dkt506. Epub 2013 Dec 30. J Antimicrob Chemother. 2014. PMID: 24381072 Free PMC article.
-
Pharmacokinetics and Pharmacodynamics of Tenofovir Reduced-Glycerin 1% Gel in the Rectal and Vaginal Compartments in Women: A Cross-Compartmental Study With Directly Observed Dosing.J Acquir Immune Defic Syndr. 2018 Jun 1;78(2):175-182. doi: 10.1097/QAI.0000000000001655. J Acquir Immune Defic Syndr. 2018. PMID: 29767639 Free PMC article. Clinical Trial.
References
-
- Anton P, et al. 2011. RMP-02/MTN-006: a phase 1, placebo-controlled trial of rectally applied 1%vaginal tenofovir gel with comparison to oral tenofovir disoproxil fumarate, abstr 34LB, p 82 Abstr. 18th Conf. Retroviruses Opportunistic Infect., Boston, MA
-
- Cicinelli E, de Ziegler D. 1999. Transvaginal progesterone: evidence for a new functional ‘portal system’ flowing from the vagina to the uterus. Hum. Reprod. Update 5:365–372 - PubMed
-
- Cohen SA. 2003. Beyond slogans: lessons from Uganda's experience with ABC and HIV/AIDS. Guttmacher Rep. Public Policy 6(5):1–3
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

