Ciliogenesis is regulated by a huntingtin-HAP1-PCM1 pathway and is altered in Huntington disease
- PMID: 21985783
- PMCID: PMC3223861
- DOI: 10.1172/JCI57552
Ciliogenesis is regulated by a huntingtin-HAP1-PCM1 pathway and is altered in Huntington disease
Abstract
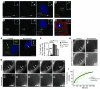
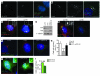
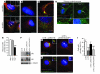
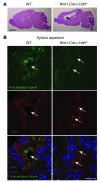
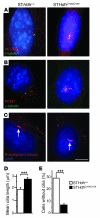
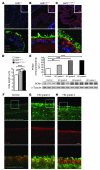
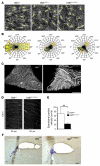
Huntington disease (HD) is a devastating autosomal-dominant neurodegenerative disorder. It is caused by expansion of a CAG repeat in the first exon of the huntingtin (HTT) gene that encodes a mutant HTT protein with a polyglutamine (polyQ) expansion at the amino terminus. Here, we demonstrate that WT HTT regulates ciliogenesis by interacting through huntingtin-associated protein 1 (HAP1) with pericentriolar material 1 protein (PCM1). Loss of Htt in mouse cells impaired the retrograde trafficking of PCM1 and thereby reduced primary cilia formation. In mice, deletion of Htt in ependymal cells led to PCM1 mislocalization, alteration of the cilia layer, and hydrocephalus. Pathogenic polyQ expansion led to centrosomal accumulation of PCM1 and abnormally long primary cilia in mouse striatal cells. PCM1 accumulation in ependymal cells was associated with longer cilia and disorganized cilia layers in a mouse model of HD and in HD patients. Longer cilia resulted in alteration of the cerebrospinal fluid flow. Thus, our data indicate that WT HTT is essential for protein trafficking to the centrosome and normal ciliogenesis. In HD, hypermorphic ciliogenesis may affect signaling and neuroblast migration so as to dysregulate brain homeostasis and exacerbate disease progression.
Figures
Comment in
-
The long and the short of aberrant ciliogenesis in Huntington disease.J Clin Invest. 2011 Nov;121(11):4237-41. doi: 10.1172/JCI60243. Epub 2011 Oct 10. J Clin Invest. 2011. PMID: 21985782 Free PMC article.
-
A huntingtin-HAP1-PCM1 pathway in ciliogenesis.Expert Rev Proteomics. 2012;9(1):17-9. doi: 10.1586/epr.11.72. Expert Rev Proteomics. 2012. PMID: 22292820 Free PMC article.
Similar articles
-
The long and the short of aberrant ciliogenesis in Huntington disease.J Clin Invest. 2011 Nov;121(11):4237-41. doi: 10.1172/JCI60243. Epub 2011 Oct 10. J Clin Invest. 2011. PMID: 21985782 Free PMC article.
-
A series of N-terminal epitope tagged Hdh knock-in alleles expressing normal and mutant huntingtin: their application to understanding the effect of increasing the length of normal Huntingtin's polyglutamine stretch on CAG140 mouse model pathogenesis.Mol Brain. 2012 Aug 14;5:28. doi: 10.1186/1756-6606-5-28. Mol Brain. 2012. PMID: 22892315 Free PMC article.
-
Aberrant splicing of HTT generates the pathogenic exon 1 protein in Huntington disease.Proc Natl Acad Sci U S A. 2013 Feb 5;110(6):2366-70. doi: 10.1073/pnas.1221891110. Epub 2013 Jan 22. Proc Natl Acad Sci U S A. 2013. PMID: 23341618 Free PMC article.
-
Polyglutamine neurodegeneration: expanded glutamines enhance native functions.Curr Opin Genet Dev. 2012 Jun;22(3):251-5. doi: 10.1016/j.gde.2012.01.001. Epub 2012 Jan 25. Curr Opin Genet Dev. 2012. PMID: 22284692 Free PMC article. Review.
-
Selective degeneration in YAC mouse models of Huntington disease.Brain Res Bull. 2007 Apr 30;72(2-3):124-31. doi: 10.1016/j.brainresbull.2006.10.018. Epub 2006 Nov 16. Brain Res Bull. 2007. PMID: 17352936 Review.
Cited by
-
Neuroanatomical and behavioral deficits in mice haploinsufficient for Pericentriolar material 1 (Pcm1).Neurosci Res. 2015 Sep;98:45-9. doi: 10.1016/j.neures.2015.02.002. Epub 2015 Feb 16. Neurosci Res. 2015. PMID: 25697395 Free PMC article.
-
Huntington's disease: the coming of age.J Genet. 2018 Jul;97(3):649-664. J Genet. 2018. PMID: 30027901 Review.
-
Ependymal Cilia: Physiology and Role in Hydrocephalus.Front Mol Neurosci. 2022 Jul 12;15:927479. doi: 10.3389/fnmol.2022.927479. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35903173 Free PMC article. Review.
-
Potential function for the Huntingtin protein as a scaffold for selective autophagy.Proc Natl Acad Sci U S A. 2014 Nov 25;111(47):16889-94. doi: 10.1073/pnas.1420103111. Epub 2014 Nov 10. Proc Natl Acad Sci U S A. 2014. PMID: 25385587 Free PMC article.
-
Genomic Instability Associated with p53 Knockdown in the Generation of Huntington's Disease Human Induced Pluripotent Stem Cells.PLoS One. 2016 Mar 16;11(3):e0150372. doi: 10.1371/journal.pone.0150372. eCollection 2016. PLoS One. 2016. PMID: 26982737 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

