Electrophysiological mapping of embryonic mouse hearts: mechanisms for developmental pacemaker switch and internodal conduction pathway
- PMID: 21985309
- PMCID: PMC3749437
- DOI: 10.1111/j.1540-8167.2011.02191.x
Electrophysiological mapping of embryonic mouse hearts: mechanisms for developmental pacemaker switch and internodal conduction pathway
Abstract
Introduction: Understanding sinoatrial node (SAN) development could help in developing therapies for SAN dysfunction. However, electrophysiological investigation of SAN development remains difficult because mutant mice with SAN dysfunctions are frequently embryonically lethal. Most research on SAN development is therefore limited to immunocytochemical observations without comparable functional studies.
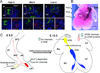
Methods and results: We applied a multielectrode array (MEA) recording system to study SAN development in mouse hearts acutely isolated at embryonic ages (E) 8.5-12.5 days. Physiological heart rates were routinely restored, enabling accurate functional assessment of SAN development. We found that dominant pacemaking activity originated from the left inflow tract (LIFT) region at E8.5, but switched to the right SAN by E12.5. Combining MEA recordings and pharmacological agents, we show that intracellular calcium (Ca(2+))-mediated automaticity develops early and is the major mechanism of pulse generation in the LIFT of E8.5 hearts. Later in development at E12.5, sarcolemmal ion channels develop in the SAN at a time when pacemaker channels are down-regulated in the LIFT, leading to a switch in the dominant pacemaker location. Additionally, low micromolar concentrations of tetrodotoxin (TTX), a sodium channel blocker, minimally affect pacemaker rhythm at E8.5-E12.5, but suppress atrial activation and reveal a TTX-resistant SAN-atrioventricular node (internodal) pathway that mediates internodal conduction in E12.5 hearts.
Conclusions: Using a physiological mapping method, we demonstrate that differential mechanistic development of automaticity between the left and right inflow tract regions confers the pacemaker location switch. Moreover, a TTX-resistant pathway mediates preferential internodal conduction in E12.5 mouse hearts.
© 2011 Wiley Periodicals, Inc.
Figures





Similar articles
-
Altered sinoatrial node function and intra-atrial conduction in murine gain-of-function Scn5a+/ΔKPQ hearts suggest an overlap syndrome.Am J Physiol Heart Circ Physiol. 2012 Apr 1;302(7):H1510-23. doi: 10.1152/ajpheart.00357.2011. Epub 2012 Jan 27. Am J Physiol Heart Circ Physiol. 2012. PMID: 22287583 Free PMC article.
-
Pacemaker activity and ionic currents in mouse atrioventricular node cells.Channels (Austin). 2011 May-Jun;5(3):241-50. doi: 10.4161/chan.5.3.15264. Epub 2011 May 1. Channels (Austin). 2011. PMID: 21406959 Free PMC article.
-
Contribution of small conductance K+ channels to sinoatrial node pacemaker activity: insights from atrial-specific Na+ /Ca2+ exchange knockout mice.J Physiol. 2017 Jun 15;595(12):3847-3865. doi: 10.1113/JP274249. Epub 2017 May 13. J Physiol. 2017. PMID: 28346695 Free PMC article.
-
Characterization of the effects of ryanodine, TTX, E-4031 and 4-AP on the sinoatrial and atrioventricular nodes.Prog Biophys Mol Biol. 2008 Jan-Apr;96(1-3):452-64. doi: 10.1016/j.pbiomolbio.2007.07.003. Epub 2007 Aug 1. Prog Biophys Mol Biol. 2008. PMID: 17850852 Review.
-
T-type channels in the sino-atrial and atrioventricular pacemaker mechanism.Pflugers Arch. 2014 Apr;466(4):791-9. doi: 10.1007/s00424-014-1482-6. Epub 2014 Feb 27. Pflugers Arch. 2014. PMID: 24573175 Review.
Cited by
-
Cardiac Morphogenesis: Specification of the Four-Chambered Heart.Cold Spring Harb Perspect Biol. 2020 Oct 1;12(10):a037143. doi: 10.1101/cshperspect.a037143. Cold Spring Harb Perspect Biol. 2020. PMID: 31932321 Free PMC article. Review.
-
Retinoic acid signaling in heart development: Application in the differentiation of cardiovascular lineages from human pluripotent stem cells.Stem Cell Reports. 2021 Nov 9;16(11):2589-2606. doi: 10.1016/j.stemcr.2021.09.010. Epub 2021 Oct 14. Stem Cell Reports. 2021. PMID: 34653403 Free PMC article. Review.
-
Development of the Cardiac Conduction System.Adv Exp Med Biol. 2024;1441:185-200. doi: 10.1007/978-3-031-44087-8_10. Adv Exp Med Biol. 2024. PMID: 38884712
-
Genetics of sinoatrial node function and heart rate disorders.Dis Model Mech. 2023 May 1;16(5):dmm050101. doi: 10.1242/dmm.050101. Epub 2023 May 17. Dis Model Mech. 2023. PMID: 37194974 Free PMC article. Review.
-
Structural and Electrical Remodeling of the Sinoatrial Node in Diabetes: New Dimensions and Perspectives.Front Endocrinol (Lausanne). 2022 Jul 7;13:946313. doi: 10.3389/fendo.2022.946313. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35872997 Free PMC article. Review.
References
-
- Schram G, Pourrier M, Melnyk P, Nattel S. Differential Distribution of Cardiac Ion Channel Expression as a Basis for Regional Specialization in Electrical Function. Circ Res. 2002;90:939–950. - PubMed
-
- Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921–1932. - PubMed
-
- Lakatta EG, Vinogradova T, Lyashkov A, Sirenko S, Zhu W, Ruknudin A, Maltsev VA. The integration of spontaneous intracellular Ca2+ cycling and surface membrane ion channel activation entrains normal automaticity in cells of the heart's pacemaker. Ann N Y Acad Sci. 2006;1080:178–206. - PubMed
-
- Barbuti A, DiFrancesco D. Control of cardiac rate by "funny" channels in health and disease. Ann N Y Acad Sci. 2008;1123:213–223. - PubMed
-
- Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993;73:197–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

