Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function
- PMID: 21985008
- PMCID: PMC3191888
- DOI: 10.1038/nmeth.1668
Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function
Abstract
Optogenetic methods have emerged as powerful tools for dissecting neural circuit connectivity, function and dysfunction. We used a bacterial artificial chromosome (BAC) transgenic strategy to express the H134R variant of channelrhodopsin-2, ChR2(H134R), under the control of cell type–specific promoter elements. We performed an extensive functional characterization of the newly established VGAT-ChR2(H134R)-EYFP, ChAT-ChR2(H134R)-EYFP, Tph2-ChR2(H134R)-EYFP and Pvalb(H134R)-ChR2-EYFP BAC transgenic mouse lines and demonstrate the utility of these lines for precisely controlling action-potential firing of GABAergic, cholinergic, serotonergic and parvalbumin-expressing neuron subsets using blue light. This resource of cell type–specific ChR2(H134R) mouse lines will facilitate the precise mapping of neuronal connectivity and the dissection of the neural basis of behavior.
Figures

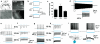
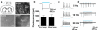
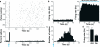
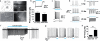

Similar articles
-
Optogenetic analysis of neuromuscular transmission in the colon of ChAT-ChR2-YFP BAC transgenic mice.Am J Physiol Gastrointest Liver Physiol. 2019 Nov 1;317(5):G569-G579. doi: 10.1152/ajpgi.00089.2019. Epub 2019 Aug 14. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31411893 Free PMC article.
-
ChAT-ChR2-EYFP mice have enhanced motor endurance but show deficits in attention and several additional cognitive domains.J Neurosci. 2013 Jun 19;33(25):10427-38. doi: 10.1523/JNEUROSCI.0395-13.2013. J Neurosci. 2013. PMID: 23785154 Free PMC article.
-
Maintenance of optogenetic channel rhodopsin (ChR2) function in aging mice: Implications for pharmacological studies of inhibitory synaptic transmission, quantal content, and calcium homeostasis.Neuropharmacology. 2023 Nov 1;238:109651. doi: 10.1016/j.neuropharm.2023.109651. Epub 2023 Jul 5. Neuropharmacology. 2023. PMID: 37414332
-
Optogenetic excitation of neurons with channelrhodopsins: light instrumentation, expression systems, and channelrhodopsin variants.Prog Brain Res. 2012;196:29-47. doi: 10.1016/B978-0-444-59426-6.00002-1. Prog Brain Res. 2012. PMID: 22341319 Review.
-
Optogenetic identification of striatal projection neuron subtypes during in vivo recordings.Brain Res. 2013 May 20;1511:21-32. doi: 10.1016/j.brainres.2012.11.018. Epub 2012 Nov 21. Brain Res. 2013. PMID: 23178332 Free PMC article. Review.
Cited by
-
Mechanics of mouse ocular motor plant quantified by optogenetic techniques.J Neurophysiol. 2015 Sep;114(3):1455-67. doi: 10.1152/jn.00328.2015. Epub 2015 Jun 24. J Neurophysiol. 2015. PMID: 26108953 Free PMC article.
-
Putative Microcircuit-Level Substrates for Attention Are Disrupted in Mouse Models of Autism.Biol Psychiatry. 2016 Apr 15;79(8):667-75. doi: 10.1016/j.biopsych.2015.04.014. Epub 2015 Apr 28. Biol Psychiatry. 2016. PMID: 26022075 Free PMC article.
-
Identification of an inhibitory circuit that regulates cerebellar Golgi cell activity.Neuron. 2012 Jan 12;73(1):149-58. doi: 10.1016/j.neuron.2011.10.030. Neuron. 2012. PMID: 22243753 Free PMC article.
-
Fatal neurological respiratory insufficiency is common among viral encephalitides.J Infect Dis. 2013 Aug 15;208(4):573-83. doi: 10.1093/infdis/jit186. Epub 2013 May 2. J Infect Dis. 2013. PMID: 23641019 Free PMC article.
-
Neurobiology of gambling behaviors.Curr Opin Neurobiol. 2013 Aug;23(4):660-7. doi: 10.1016/j.conb.2013.03.004. Epub 2013 Mar 29. Curr Opin Neurobiol. 2013. PMID: 23541597 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

