Two non-vesicular ATP release pathways in the mouse erythrocyte membrane
- PMID: 21983290
- PMCID: PMC3218561
- DOI: 10.1016/j.febslet.2011.09.033
Two non-vesicular ATP release pathways in the mouse erythrocyte membrane
Abstract
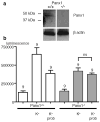
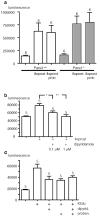
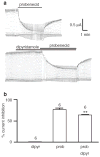
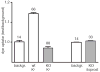
Erythrocytes are exceptionally suited for analysis of non-exocytotic release mechanisms of ATP, because these cells under physiological conditions lack vesicles. Previous studies have indicated, that Pannexin1 (Panx1) provides a key ATP permeation pathway in many cell types, including human and frog erythrocytes. Here we show that erythrocytes of Panx1(-/-) mice lend further support to this conclusion. However, ATP release, although attenuated, was still observed in Panx1(-/-) mouse erythrocytes. In contrast to Panx1(+/+) cells, this release was not correlated with uptake of extracellularly applied dyes, was insensitive to Panx1 channel blockers, and was inhibited by dipyridamole and stimulated by iloprost. Thus, in erythrocytes, two independent pathways mediate the release of ATP. We also show that glyburide is a strong inhibitor of Panx1 channels.
Copyright © 2011 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Pannexin1 channels act downstream of P2X 7 receptors in ATP-induced murine T-cell death.Channels (Austin). 2014;8(2):142-56. doi: 10.4161/chan.28122. Epub 2014 Mar 3. Channels (Austin). 2014. PMID: 24590064 Free PMC article.
-
Prostacyclin receptor-mediated ATP release from erythrocytes requires the voltage-dependent anion channel.Am J Physiol Heart Circ Physiol. 2012 Feb 1;302(3):H553-9. doi: 10.1152/ajpheart.00998.2011. Epub 2011 Dec 9. Am J Physiol Heart Circ Physiol. 2012. PMID: 22159995 Free PMC article.
-
Pannexin1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated free fatty acids in liver cells.Am J Physiol Cell Physiol. 2012 Nov 15;303(10):C1034-44. doi: 10.1152/ajpcell.00175.2012. Epub 2012 Sep 12. Am J Physiol Cell Physiol. 2012. PMID: 22972801 Free PMC article.
-
Pannexin: from discovery to bedside in 11±4 years?Brain Res. 2012 Dec 3;1487:150-9. doi: 10.1016/j.brainres.2012.04.058. Epub 2012 Jul 4. Brain Res. 2012. PMID: 22771709 Free PMC article. Review.
-
[Distribution and regulation of Panx1 protein].Sheng Li Ke Xue Jin Zhan. 2013 Jun;44(3):188-92. Sheng Li Ke Xue Jin Zhan. 2013. PMID: 24027825 Review. Chinese.
Cited by
-
Dynamic Regulation of Cell Volume and Extracellular ATP of Human Erythrocytes.PLoS One. 2016 Jun 29;11(6):e0158305. doi: 10.1371/journal.pone.0158305. eCollection 2016. PLoS One. 2016. PMID: 27355484 Free PMC article.
-
A comparative antibody analysis of pannexin1 expression in four rat brain regions reveals varying subcellular localizations.Front Pharmacol. 2013 Feb 6;4:6. doi: 10.3389/fphar.2013.00006. eCollection 2013. Front Pharmacol. 2013. PMID: 23390418 Free PMC article.
-
Mechanosensitive Pannexin 1 Activity Is Modulated by Stomatin in Human Red Blood Cells.Int J Mol Sci. 2022 Aug 20;23(16):9401. doi: 10.3390/ijms23169401. Int J Mol Sci. 2022. PMID: 36012667 Free PMC article.
-
Physiological mechanisms for the modulation of pannexin 1 channel activity.J Physiol. 2012 Dec 15;590(24):6257-66. doi: 10.1113/jphysiol.2012.240911. Epub 2012 Oct 15. J Physiol. 2012. PMID: 23070703 Free PMC article. Review.
-
Trovafloxacin attenuates neuroinflammation and improves outcome after traumatic brain injury in mice.J Neuroinflammation. 2018 Feb 13;15(1):42. doi: 10.1186/s12974-018-1069-9. J Neuroinflammation. 2018. PMID: 29439712 Free PMC article.
References
-
- Burnstock G. Overview Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–17. discussion 18. - PubMed
-
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. - PubMed
-
- Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–7. - PubMed
-
- Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

