The differential role of Hif1β/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation
- PMID: 21982709
- PMCID: PMC3206000
- DOI: 10.1016/j.cmet.2011.08.006
The differential role of Hif1β/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation
Abstract
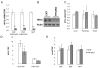
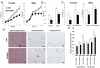
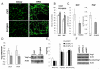
In obesity, adipocytes distant from vasculature become hypoxic and dysfunctional. This hypoxic response is mediated by hypoxia-inducible factors (Hif1α, Hif2α, and Hif3α) and their obligate partner, Hif1β (Arnt). We show that mice lacking Hif1β in fat (FH1βKO) are lean, exhibit reduced adipocyte size, and are protected from age- and diet-induced glucose intolerance. There is also reduced Vegf and vascular permeability in FH1βKO fat, but diet-induced inflammation and fibrosis is unchanged. Adipocytes from FH1βKO mice have reduced glucose uptake due to decreased Glut1 and Glut4, which is mirrored in 3T3-L1 adipocytes with Hif1β knockdown. Hif1β knockdown cells also fail to respond appropriately to hypoxia with reduced cellular respiration and reduced mitochondrial gene expression. Some, but not all, of these effects are reproduced by Hif1α knockdown. Thus, Hif1β/Arnt regulates glucose uptake, mitochondrial gene expression, and vascular permeability to control adipose mass and function, providing a target for obesity therapy.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

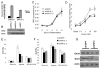

Similar articles
-
Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice.Diabetes. 2011 Oct;60(10):2484-95. doi: 10.2337/db11-0174. Epub 2011 Aug 26. Diabetes. 2011. PMID: 21873554 Free PMC article.
-
The Loss of ARNT/HIF1β in Male Pancreatic β-Cells Is Protective Against High-Fat Diet-Induced Diabetes.Endocrinology. 2019 Dec 1;160(12):2825-2836. doi: 10.1210/en.2018-00936. Endocrinology. 2019. PMID: 31580427 Free PMC article.
-
Deletion of ARNT/HIF1β in pancreatic beta cells does not impair glucose homeostasis in mice, but is associated with defective glucose sensing ex vivo.Diabetologia. 2015 Dec;58(12):2832-42. doi: 10.1007/s00125-015-3768-4. Epub 2015 Sep 26. Diabetologia. 2015. PMID: 26409461 Free PMC article.
-
Muscle Arnt/Hif1β Is Dispensable in Myofiber Type Determination, Vascularization and Insulin Sensitivity.PLoS One. 2016 Dec 22;11(12):e0168457. doi: 10.1371/journal.pone.0168457. eCollection 2016. PLoS One. 2016. PMID: 28005939 Free PMC article.
-
Cellular hypoxia and adipose tissue dysfunction in obesity.Proc Nutr Soc. 2009 Nov;68(4):370-7. doi: 10.1017/S0029665109990206. Epub 2009 Aug 24. Proc Nutr Soc. 2009. PMID: 19698203 Review.
Cited by
-
Adipose tissue angiogenesis: impact on obesity and type-2 diabetes.Biochim Biophys Acta. 2014 Mar;1842(3):463-72. doi: 10.1016/j.bbadis.2013.06.003. Epub 2013 Jun 12. Biochim Biophys Acta. 2014. PMID: 23770388 Free PMC article. Review.
-
Hypoxia-regulated mechanisms in the pathogenesis of obesity and non-alcoholic fatty liver disease.Cell Mol Life Sci. 2016 Sep;73(18):3419-31. doi: 10.1007/s00018-016-2222-1. Epub 2016 Apr 18. Cell Mol Life Sci. 2016. PMID: 27091156 Free PMC article. Review.
-
Hepatocyte-specific HIF-1α ablation improves obesity-induced glucose intolerance by reducing first-pass GLP-1 degradation.Sci Adv. 2019 Jul 3;5(7):eaaw4176. doi: 10.1126/sciadv.aaw4176. eCollection 2019 Jul. Sci Adv. 2019. PMID: 31281892 Free PMC article.
-
Hypoxia-Inducible Factors and the Regulation of Lipid Metabolism.Cells. 2019 Mar 3;8(3):214. doi: 10.3390/cells8030214. Cells. 2019. PMID: 30832409 Free PMC article. Review.
-
Tbx15 Defines a Glycolytic Subpopulation and White Adipocyte Heterogeneity.Diabetes. 2017 Nov;66(11):2822-2829. doi: 10.2337/db17-0218. Epub 2017 Aug 28. Diabetes. 2017. PMID: 28847884 Free PMC article.
References
-
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. - PubMed
-
- Alexander DL, Ganem LG, Fernandez-Salguero P, Gonzalez F, Jefcoate CR. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci. 1998;111(Pt 22):3311–3322. - PubMed
-
- Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–9. - PubMed
-
- Bluher M, Patti ME, Gesta S, Kahn BB, Kahn CR. Intrinsic heterogeneity in adipose tissue of fat-specific insulin receptor knock-out mice is associated with differences in patterns of gene expression. J Biol Chem. 2004;279:31891–31901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

