Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction
- PMID: 21982706
- PMCID: PMC3235745
- DOI: 10.1016/j.cmet.2011.08.009
Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction
Erratum in
-
Distinct Hypothalamic Neurons Mediate Estrogenic Effects on Energy Homeostasis and Reproduction.Cell Metab. 2019 May 7;29(5):1232. doi: 10.1016/j.cmet.2019.04.006. Cell Metab. 2019. PMID: 31067449 Free PMC article. No abstract available.
Abstract
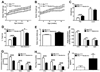
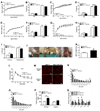
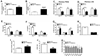
Estrogens regulate body weight and reproduction primarily through actions on estrogen receptor-α (ERα). However, ERα-expressing cells mediating these effects are not identified. We demonstrate that brain-specific deletion of ERα in female mice causes abdominal obesity stemming from both hyperphagia and hypometabolism. Hypometabolism and abdominal obesity, but not hyperphagia, are recapitulated in female mice lacking ERα in hypothalamic steroidogenic factor-1 (SF1) neurons. In contrast, deletion of ERα in hypothalamic pro-opiomelanocortin (POMC) neurons leads to hyperphagia, without directly influencing energy expenditure or fat distribution. Further, simultaneous deletion of ERα from both SF1 and POMC neurons causes hypometabolism, hyperphagia, and increased visceral adiposity. Additionally, female mice lacking ERα in SF1 neurons develop anovulation and infertility, while POMC-specific deletion of ERα inhibits negative feedback regulation of estrogens and impairs fertility in females. These results indicate that estrogens act on distinct hypothalamic ERα neurons to regulate different aspects of energy homeostasis and reproduction.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Estrogens and obesity: is it all in our heads?Cell Metab. 2011 Oct 5;14(4):435-6. doi: 10.1016/j.cmet.2011.09.003. Cell Metab. 2011. PMID: 21982701 Free PMC article.
Similar articles
-
Developmental changes in hypothalamic SF-1, POMC, and ERα mRNA expression and their sensitivity to fasting in male and female rats.Endocr J. 2017 Dec 28;64(12):1157-1163. doi: 10.1507/endocrj.EJ17-0140. Epub 2017 Sep 6. Endocr J. 2017. PMID: 28883210
-
Female Mice Lacking Estrogen Receptor-α in Hypothalamic Proopiomelanocortin (POMC) Neurons Display Enhanced Estrogenic Response on Cortical Bone Mass.Endocrinology. 2016 Aug;157(8):3242-52. doi: 10.1210/en.2016-1181. Epub 2016 Jun 2. Endocrinology. 2016. PMID: 27254004 Free PMC article.
-
Steroid receptor coactivator-1 mediates estrogenic actions to prevent body weight gain in female mice.Endocrinology. 2013 Jan;154(1):150-8. doi: 10.1210/en.2012-2007. Epub 2012 Dec 4. Endocrinology. 2013. PMID: 23211707 Free PMC article.
-
Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor.Steroids. 2008 Oct;73(9-10):985-91. doi: 10.1016/j.steroids.2007.11.008. Epub 2008 Feb 9. Steroids. 2008. PMID: 18342349 Free PMC article. Review.
-
New Insights of SF1 Neurons in Hypothalamic Regulation of Obesity and Diabetes.Int J Mol Sci. 2021 Jun 8;22(12):6186. doi: 10.3390/ijms22126186. Int J Mol Sci. 2021. PMID: 34201257 Free PMC article. Review.
Cited by
-
Wiring the Brain for Wellness: Sensory Integration in Feeding and Thermogenesis: A Report on Research Supported by Pathway to Stop Diabetes.Diabetes. 2024 Mar 1;73(3):338-347. doi: 10.2337/db23-0706. Diabetes. 2024. PMID: 38377445 Free PMC article. Review.
-
Multiple estrogen receptor subtypes influence ingestive behavior in female rodents.Physiol Behav. 2015 Dec 1;152(Pt B):431-7. doi: 10.1016/j.physbeh.2015.05.032. Epub 2015 May 31. Physiol Behav. 2015. PMID: 26037634 Free PMC article. Review.
-
Nuclear Factor Erythroid 2-Related Factor 2 Deficiency Results in Amplification of the Liver Fat-Lowering Effect of Estrogen.J Pharmacol Exp Ther. 2016 Jul;358(1):14-21. doi: 10.1124/jpet.115.231316. Epub 2016 May 9. J Pharmacol Exp Ther. 2016. PMID: 27189962 Free PMC article.
-
Hypothalamic AMPK: a canonical regulator of whole-body energy balance.Nat Rev Endocrinol. 2016 Jul;12(7):421-32. doi: 10.1038/nrendo.2016.67. Epub 2016 May 20. Nat Rev Endocrinol. 2016. PMID: 27199291 Review.
-
SF1-Specific AMPKα1 Deletion Protects Against Diet-Induced Obesity.Diabetes. 2018 Nov;67(11):2213-2226. doi: 10.2337/db17-1538. Epub 2018 Aug 13. Diabetes. 2018. PMID: 30104247 Free PMC article.
References
-
- Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2007;148:5656–5666. - PubMed
-
- Billeci AM, Paciaroni M, Caso V, Agnelli G. Hormone replacement therapy and stroke. Curr Vasc Pharmacol. 2008;6:112–123. - PubMed
-
- Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. - PubMed
-
- Castaneda TR, Jurgens H, Wiedmer P, Pfluger P, Diano S, Horvath TL, Tang-Christensen M, Tschop MH. Obesity and the neuroendocrine control of energy homeostasis: the role of spontaneous locomotor activity. J Nutr. 2005;135:1314–1319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD061539/HD/NICHD NIH HHS/United States
- R00 DK085330/DK/NIDDK NIH HHS/United States
- R37 DK053301/DK/NIDDK NIH HHS/United States
- HD061539/HD/NICHD NIH HHS/United States
- R01 DK073689-01A1/DK/NIDDK NIH HHS/United States
- P30 DK079638/DK/NIDDK NIH HHS/United States
- DK073689/DK/NIDDK NIH HHS/United States
- R01 DK093587/DK/NIDDK NIH HHS/United States
- R00DK085330/DK/NIDDK NIH HHS/United States
- R37DK53301/DK/NIDDK NIH HHS/United States
- R01DK071320/DK/NIDDK NIH HHS/United States
- R01 DK073689/DK/NIDDK NIH HHS/United States
- P01 DK088761/DK/NIDDK NIH HHS/United States
- R01DK093587/DK/NIDDK NIH HHS/United States
- R01DK088423/DK/NIDDK NIH HHS/United States
- RL1 DK081185/DK/NIDDK NIH HHS/United States
- P01DK088761/DK/NIDDK NIH HHS/United States
- P30 DK079638-03/DK/NIDDK NIH HHS/United States
- R01 DK088423/DK/NIDDK NIH HHS/United States
- UL1 RR024923/RR/NCRR NIH HHS/United States
- PL1 DK081182/DK/NIDDK NIH HHS/United States
- DK088220/DK/NIDDK NIH HHS/United States
- R01 DK071320/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

