A syndecan-4 hair trigger initiates wound healing through caveolin- and RhoG-regulated integrin endocytosis
- PMID: 21982645
- PMCID: PMC3202633
- DOI: 10.1016/j.devcel.2011.08.007
A syndecan-4 hair trigger initiates wound healing through caveolin- and RhoG-regulated integrin endocytosis
Erratum in
- Dev Cell. 2012 Nov 13;23(5):1081-2
Abstract
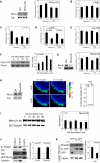
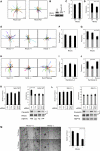
Cell migration during wound healing requires adhesion receptor turnover to enable the formation and disassembly of cell-extracellular matrix contacts. Although recent advances have improved our understanding of integrin trafficking pathways, it is not known how extracellular ligand engagement controls receptor dynamics. Using atomic force microscopy, we have measured cell avidity for fibronectin and defined a mechanism for the outside-in regulation of α(5)β(1)-integrin. Surprisingly, adhesive strength was attenuated by the syndecan-4-binding domain of fibronectin due to a rapid triggering of α(5)β(1)-integrin endocytosis. Association of syndecan-4 with PKCα was found to trigger RhoG activation and subsequent dynamin- and caveolin-dependent integrin uptake. Like disruption of syndecan-4 or caveolin, gene disruption of RhoG in mice was found to retard closure of dermal wounds due to a migration defect of the fibroblasts and keratinocytes of RhoG null mice. Thus, this syndecan-4-regulated integrin endocytic pathway appears to play a key role in tissue repair.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
RGD-independent cell adhesion via a tissue transglutaminase-fibronectin matrix promotes fibronectin fibril deposition and requires syndecan-4/2 α5β1 integrin co-signaling.J Biol Chem. 2010 Dec 17;285(51):40212-29. doi: 10.1074/jbc.M110.123703. Epub 2010 Oct 7. J Biol Chem. 2010. PMID: 20929862 Free PMC article.
-
Therapeutic ultrasound bypasses canonical syndecan-4 signaling to activate rac1.J Biol Chem. 2009 Mar 27;284(13):8898-909. doi: 10.1074/jbc.M804281200. Epub 2009 Jan 15. J Biol Chem. 2009. PMID: 19147498 Free PMC article.
-
Syndecan-4 independently regulates multiple small GTPases to promote fibroblast migration during wound healing.Small GTPases. 2012 Apr-Jun;3(2):73-9. doi: 10.4161/sgtp.19301. Small GTPases. 2012. PMID: 22790193 Free PMC article.
-
Cytoplasmic interactions of syndecan-4 orchestrate adhesion receptor and growth factor receptor signalling.Biochem J. 2002 Nov 15;368(Pt 1):1-15. doi: 10.1042/BJ20021228. Biochem J. 2002. PMID: 12241528 Free PMC article. Review.
-
Syndecan-4 signaling at a glance.J Cell Sci. 2013 Sep 1;126(Pt 17):3799-804. doi: 10.1242/jcs.124636. Epub 2013 Aug 22. J Cell Sci. 2013. PMID: 23970415 Free PMC article. Review.
Cited by
-
Basal endothelial glycocalyx's response to shear stress: a review of structure, function, and clinical implications.Front Cell Dev Biol. 2024 Mar 18;12:1371769. doi: 10.3389/fcell.2024.1371769. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38562144 Free PMC article. Review.
-
Thy-1 (CD90)-regulated cell adhesion and migration of mesenchymal cells: insights into adhesomes, mechanical forces, and signaling pathways.Front Cell Dev Biol. 2023 Nov 30;11:1221306. doi: 10.3389/fcell.2023.1221306. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38099295 Free PMC article. Review.
-
Systemically administered wound-homing peptide accelerates wound healing by modulating syndecan-4 function.Nat Commun. 2023 Dec 6;14(1):8069. doi: 10.1038/s41467-023-43848-1. Nat Commun. 2023. PMID: 38057316 Free PMC article.
-
Effects of Heparan sulfate acetyl-CoA: Alpha-glucosaminide N-acetyltransferase (HGSNAT) inactivation on the structure and function of epithelial and immune cells of the testis and epididymis and sperm parameters in adult mice.PLoS One. 2023 Sep 27;18(9):e0292157. doi: 10.1371/journal.pone.0292157. eCollection 2023. PLoS One. 2023. PMID: 37756356 Free PMC article.
-
Engineered Biomimetic Fibrillar Fibronectin Matrices Regulate Cell Adhesion Initiation, Migration, and Proliferation via α5β1 Integrin and Syndecan-4 Crosstalk.Adv Sci (Weinh). 2023 Aug;10(24):e2300812. doi: 10.1002/advs.202300812. Epub 2023 Jun 25. Adv Sci (Weinh). 2023. PMID: 37357136 Free PMC article.
References
-
- Cao H., Courchesne W.E., Mastick C.C. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J. Biol. Chem. 2002;277:8771–8774. - PubMed
-
- Caswell P.T., Norman J.C. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

