Quantitative in vivo analyses reveal calcium-dependent phosphorylation sites and identifies a novel component of the Toxoplasma invasion motor complex
- PMID: 21980283
- PMCID: PMC3182922
- DOI: 10.1371/journal.ppat.1002222
Quantitative in vivo analyses reveal calcium-dependent phosphorylation sites and identifies a novel component of the Toxoplasma invasion motor complex
Abstract
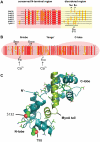
Apicomplexan parasites depend on the invasion of host cells for survival and proliferation. Calcium-dependent signaling pathways appear to be essential for micronemal release and gliding motility, yet the target of activated kinases remains largely unknown. We have characterized calcium-dependent phosphorylation events during Toxoplasma host cell invasion. Stimulation of live tachyzoites with Ca²⁺-mobilizing drugs leads to phosphorylation of numerous parasite proteins, as shown by differential 2-DE display of ³²[P]-labeled protein extracts. Multi-dimensional Protein Identification Technology (MudPIT) identified ∼546 phosphorylation sites on over 300 Toxoplasma proteins, including 10 sites on the actomyosin invasion motor. Using a Stable Isotope of Amino Acids in Culture (SILAC)-based quantitative LC-MS/MS analyses we monitored changes in the abundance and phosphorylation of the invasion motor complex and defined Ca²⁺-dependent phosphorylation patterns on three of its components--GAP45, MLC1 and MyoA. Furthermore, calcium-dependent phosphorylation of six residues across GAP45, MLC1 and MyoA is correlated with invasion motor activity. By analyzing proteins that appear to associate more strongly with the invasion motor upon calcium stimulation we have also identified a novel 15-kDa Calmodulin-like protein that likely represents the MyoA Essential Light Chain of the Toxoplasma invasion motor. This suggests that invasion motor activity could be regulated not only by phosphorylation but also by the direct binding of calcium ions to this new component.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
GAP45 phosphorylation controls assembly of the Toxoplasma myosin XIV complex.Eukaryot Cell. 2009 Feb;8(2):190-6. doi: 10.1128/EC.00201-08. Epub 2008 Dec 1. Eukaryot Cell. 2009. PMID: 19047362 Free PMC article.
-
The toxoplasma Acto-MyoA motor complex is important but not essential for gliding motility and host cell invasion.PLoS One. 2014 Mar 14;9(3):e91819. doi: 10.1371/journal.pone.0091819. eCollection 2014. PLoS One. 2014. PMID: 24632839 Free PMC article.
-
Two Essential Light Chains Regulate the MyoA Lever Arm To Promote Toxoplasma Gliding Motility.mBio. 2015 Sep 15;6(5):e00845-15. doi: 10.1128/mBio.00845-15. mBio. 2015. PMID: 26374117 Free PMC article.
-
Calcium signaling and the lytic cycle of the Apicomplexan parasite Toxoplasma gondii.Biochim Biophys Acta Mol Cell Res. 2018 Nov;1865(11 Pt B):1846-1856. doi: 10.1016/j.bbamcr.2018.08.004. Epub 2018 Aug 10. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 30992126 Free PMC article. Review.
-
Environmental sensing and regulation of motility in Toxoplasma.Mol Microbiol. 2021 May;115(5):916-929. doi: 10.1111/mmi.14661. Epub 2020 Dec 28. Mol Microbiol. 2021. PMID: 33278047 Review.
Cited by
-
A Toxoplasma palmitoyl acyl transferase and the palmitoylated armadillo repeat protein TgARO govern apical rhoptry tethering and reveal a critical role for the rhoptries in host cell invasion but not egress.PLoS Pathog. 2013 Feb;9(2):e1003162. doi: 10.1371/journal.ppat.1003162. Epub 2013 Feb 7. PLoS Pathog. 2013. PMID: 23408890 Free PMC article.
-
Global proteomic analysis of the oocyst/sporozoite of Toxoplasma gondii reveals commitment to a host-independent lifestyle.BMC Genomics. 2013 Mar 15;14:183. doi: 10.1186/1471-2164-14-183. BMC Genomics. 2013. PMID: 23496850 Free PMC article.
-
The apical complex provides a regulated gateway for secretion of invasion factors in Toxoplasma.PLoS Pathog. 2014 Apr 17;10(4):e1004074. doi: 10.1371/journal.ppat.1004074. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24743791 Free PMC article.
-
MyosinA is a druggable target in the widespread protozoan parasite Toxoplasma gondii.PLoS Biol. 2023 May 8;21(5):e3002110. doi: 10.1371/journal.pbio.3002110. eCollection 2023 May. PLoS Biol. 2023. PMID: 37155705 Free PMC article.
-
A Toxoplasma gondii class XIV myosin, expressed in Sf9 cells with a parasite co-chaperone, requires two light chains for fast motility.J Biol Chem. 2014 Oct 31;289(44):30832-30841. doi: 10.1074/jbc.M114.572453. Epub 2014 Sep 17. J Biol Chem. 2014. PMID: 25231988 Free PMC article.
References
-
- de Carvalho KM, Minguini N, Moreira Filho DC, Kara-Jose N. Characteristics of a pediatric low-vision population. J Pediatr Ophthalmol Strabismus. 1998;35:162–165. - PubMed
-
- Meissner M, Schluter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298:837–840. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

