Levels of BDNF impact oligodendrocyte lineage cells following a cuprizone lesion
- PMID: 21976503
- PMCID: PMC3203635
- DOI: 10.1523/JNEUROSCI.6595-10.2011
Levels of BDNF impact oligodendrocyte lineage cells following a cuprizone lesion
Abstract
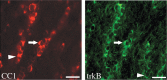
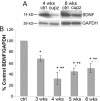
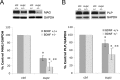
Previous work in culture has shown that basal forebrain (BF) oligodendrocyte (OLG) lineage cells respond to BDNF by increasing DNA synthesis and differentiation. Further, in the BF in vivo, reduced levels of BDNF as seen in BDNF(+/-) mice result in reduced numbers of NG2+ cells and deficits in myelin proteins throughout development and in the adult, suggesting that BDNF impacts the proliferating population of OLGs as well as differentiation in vivo. In this study, to investigate the roles BDNF may play in the repair of a demyelinating lesion, the cuprizone model was used and the corpus callosum was examined. BDNF protein levels were reduced after cuprizone treatment, suggesting that the demyelinating lesion itself elicits a decrease in BDNF. To analyze the effects of a further reduction of BDNF on OLG lineage cells following cuprizone, BDNF(+/-) mice were evaluated. These mice exhibited a blunted increase in the NG2 response at 4 and 5 weeks of cuprizone treatment. In addition, BDNF(+/-) mice exhibited decreased levels of myelin proteins during the demyelination and remyelination processes with no change in the total number of OLGs. These effects appear to be relatively specific to OLG lineage cells as comparable changes in CD11b+ microglia, GFAP+ astrocytes, and SMI32+ injured axons were not observed. These data indicate that BDNF may play a role following a demyelinating lesion by regulating the numbers of progenitors and the abilities of demyelinating and differentiating cells to express myelin proteins.
Figures




Similar articles
-
Tropomyosin Receptor Kinase B Expressed in Oligodendrocyte Lineage Cells Functions to Promote Myelin Following a Demyelinating Lesion.ASN Neuro. 2020 Jan-Dec;12:1759091420957464. doi: 10.1177/1759091420957464. ASN Neuro. 2020. PMID: 32927995 Free PMC article.
-
Brain-derived neurotrophic factor deficiency restricts proliferation of oligodendrocyte progenitors following cuprizone-induced demyelination.ASN Neuro. 2015 Jan 13;7(1):1759091414566878. doi: 10.1177/1759091414566878. Print 2015 Jan-Feb. ASN Neuro. 2015. PMID: 25586993 Free PMC article.
-
Icariin ameliorates the cuprizone-induced acute brain demyelination and modulates the number of oligodendrocytes, microglia and astrocytes in the brain of C57BL/6J mice.Brain Res Bull. 2021 Oct;175:37-47. doi: 10.1016/j.brainresbull.2021.07.010. Epub 2021 Jul 15. Brain Res Bull. 2021. PMID: 34274431
-
BDNF+/- mice exhibit deficits in oligodendrocyte lineage cells of the basal forebrain.Glia. 2010 May;58(7):848-56. doi: 10.1002/glia.20969. Glia. 2010. PMID: 20091777 Free PMC article.
-
The role of growth factors as a therapeutic approach to demyelinating disease.Exp Neurol. 2016 Sep;283(Pt B):531-40. doi: 10.1016/j.expneurol.2016.02.023. Epub 2016 Mar 22. Exp Neurol. 2016. PMID: 27016070 Free PMC article. Review.
Cited by
-
Pharmacological approaches to intervention in hypomyelinating and demyelinating white matter pathology.Neuropharmacology. 2016 Nov;110(Pt B):605-625. doi: 10.1016/j.neuropharm.2015.06.008. Epub 2015 Jun 24. Neuropharmacology. 2016. PMID: 26116759 Free PMC article. Review.
-
2,4 Dinitrophenol as Medicine.Cells. 2019 Mar 23;8(3):280. doi: 10.3390/cells8030280. Cells. 2019. PMID: 30909602 Free PMC article. Review.
-
The neuroprotective effects of Chalcones from Ashitaba on cuprizone-induced demyelination via modulation of brain-derived neurotrophic factor and tumor necrosis factor α.Brain Behav. 2023 Sep;13(9):e3144. doi: 10.1002/brb3.3144. Epub 2023 Jul 4. Brain Behav. 2023. PMID: 37403256 Free PMC article.
-
Experimental Autoimmune Encephalomyelitis (EAE)-Induced Elevated Expression of the E1 Isoform of Methyl CpG Binding Protein 2 (MeCP2E1): Implications in Multiple Sclerosis (MS)-Induced Neurological Disability and Associated Myelin Damage.Int J Mol Sci. 2017 Jun 12;18(6):1254. doi: 10.3390/ijms18061254. Int J Mol Sci. 2017. PMID: 28604632 Free PMC article.
-
BDNF-TrkB Signaling Pathway in Spinal Cord Injury: Insights and Implications.Mol Neurobiol. 2025 Feb;62(2):1904-1944. doi: 10.1007/s12035-024-04381-4. Epub 2024 Jul 24. Mol Neurobiol. 2025. PMID: 39046702 Review.
References
-
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. - PubMed
-
- Althaus HH. Remyelination in multiple sclerosis: a new role for neurotrophins? Prog Brain Res. 2004;146:415–432. - PubMed
-
- Azoulay D, Vachapova V, Shihman B, Miler A, Karni A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. J Neuroimmunol. 2005;167:215–218. - PubMed
-
- Biancotti JC, Kumar S, de Vellis J. Activation of inflammatory response by a combination of growth factors in cuprizone-induced demyelinated brain leads to myelin repair. Neurochem Res. 2008;33:2615–2628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
