Estimating treatment effects for individual patients based on the results of randomised clinical trials
- PMID: 21968126
- PMCID: PMC3184644
- DOI: 10.1136/bmj.d5888
Estimating treatment effects for individual patients based on the results of randomised clinical trials
Abstract
Objectives: To predict treatment effects for individual patients based on data from randomised trials, taking rosuvastatin treatment in the primary prevention of cardiovascular disease as an example, and to evaluate the net benefit of making treatment decisions for individual patients based on a predicted absolute treatment effect.
Setting: As an example, data were used from the Justification for the Use of Statins in Prevention (JUPITER) trial, a randomised controlled trial evaluating the effect of rosuvastatin 20 mg daily versus placebo on the occurrence of cardiovascular events (myocardial infarction, stroke, arterial revascularisation, admission to hospital for unstable angina, or death from cardiovascular causes). Population 17,802 healthy men and women who had low density lipoprotein cholesterol levels of less than 3.4 mmol/L and high sensitivity C reactive protein levels of 2.0 mg/L or more.
Methods: Data from the Justification for the Use of Statins in Prevention trial were used to predict rosuvastatin treatment effect for individual patients based on existing risk scores (Framingham and Reynolds) and on a newly developed prediction model. We compared the net benefit of prediction based rosuvastatin treatment (selective treatment of patients whose predicted treatment effect exceeds a decision threshold) with the net benefit of treating either everyone or no one.
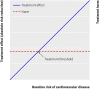
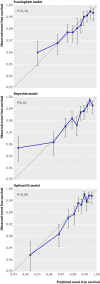
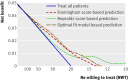
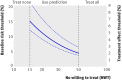
Results: The median predicted 10 year absolute risk reduction for cardiovascular events was 4.4% (interquartile range 2.6-7.0%) based on the Framingham risk score, 4.2% (2.5-7.1%) based on the Reynolds score, and 3.9% (2.5-6.1%) based on the newly developed model (optimal fit model). Prediction based treatment was associated with more net benefit than treating everyone or no one, provided that the decision threshold was between 2% and 7%, and thus that the number willing to treat (NWT) to prevent one cardiovascular event over 10 years was between 15 and 50.
Conclusions: Data from randomised trials can be used to predict treatment effect in terms of absolute risk reduction for individual patients, based on a newly developed model or, if available, existing risk scores. The value of such prediction of treatment effect for medical decision making is conditional on the NWT to prevent one outcome event. Trial registration number Clinicaltrials.gov NCT00239681.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures

Similar articles
-
Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER).Circ Cardiovasc Qual Outcomes. 2009 Nov;2(6):616-23. doi: 10.1161/CIRCOUTCOMES.109.848473. Epub 2009 Sep 22. Circ Cardiovasc Qual Outcomes. 2009. PMID: 20031900 Clinical Trial.
-
Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial.Lancet. 2009 Apr 4;373(9670):1175-82. doi: 10.1016/S0140-6736(09)60447-5. Epub 2009 Mar 28. Lancet. 2009. PMID: 19329177 Clinical Trial.
-
Kinesin-like protein 6 (KIF6) polymorphism and the efficacy of rosuvastatin in primary prevention.Circ Cardiovasc Genet. 2011 Jun;4(3):312-7. doi: 10.1161/CIRCGENETICS.110.959353. Epub 2011 Apr 14. Circ Cardiovasc Genet. 2011. PMID: 21493817
-
Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)--can C-reactive protein be used to target statin therapy in primary prevention?Am J Cardiol. 2006 Jan 16;97(2A):33A-41A. doi: 10.1016/j.amjcard.2005.11.014. Epub 2005 Dec 1. Am J Cardiol. 2006. PMID: 16442935 Review.
-
Primary prevention of coronary heart disease: integration of new data, evolving views, revised goals, and role of rosuvastatin in management. A comprehensive survey.Drug Des Devel Ther. 2011;5:325-80. doi: 10.2147/DDDT.S14934. Epub 2011 Jun 13. Drug Des Devel Ther. 2011. PMID: 21792295 Free PMC article. Review.
Cited by
-
Personalized prediction of lifetime benefits with statin therapy for asymptomatic individuals: a modeling study.PLoS Med. 2012;9(12):e1001361. doi: 10.1371/journal.pmed.1001361. Epub 2012 Dec 27. PLoS Med. 2012. PMID: 23300388 Free PMC article.
-
Machine learning-based prediction model for the efficacy and safety of statins.Front Pharmacol. 2024 Jul 29;15:1334929. doi: 10.3389/fphar.2024.1334929. eCollection 2024. Front Pharmacol. 2024. PMID: 39135800 Free PMC article.
-
IMproving the imPlemEntation of cuRrent guidelines for the mAnagement of major coronary hearT disease rIsk factors by multifactorial interVEntion. The IMPERATIVE renal analysis.Arch Med Sci. 2011 Dec 31;7(6):984-92. doi: 10.5114/aoms.2011.26610. Epub 2011 Dec 30. Arch Med Sci. 2011. PMID: 22328881 Free PMC article.
-
Development and external validation of a clinical prognostic score for death in visceral leishmaniasis patients in a high HIV co-infection burden area in Ethiopia.PLoS One. 2017 Jun 5;12(6):e0178996. doi: 10.1371/journal.pone.0178996. eCollection 2017. PLoS One. 2017. PMID: 28582440 Free PMC article.
-
Support of personalized medicine through risk-stratified treatment recommendations - an environmental scan of clinical practice guidelines.BMC Med. 2013 Jan 9;11:7. doi: 10.1186/1741-7015-11-7. BMC Med. 2013. PMID: 23302096 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
