External Ba2+ block of the two-pore domain potassium channel TREK-1 defines conformational transition in its selectivity filter
- PMID: 21965685
- PMCID: PMC3220548
- DOI: 10.1074/jbc.M111.264788
External Ba2+ block of the two-pore domain potassium channel TREK-1 defines conformational transition in its selectivity filter
Abstract
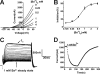
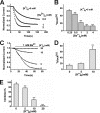
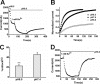
TREK-1 is a member of the two-pore domain potassium channel family that is known as a leak channel and plays a key role in many physiological and pathological processes. The conformational transition of the selectivity filter is considered as an effective strategy for potassium channels to control the course of potassium efflux. It is well known that TREK-1 is regulated by a large volume of extracellular and intracellular signals. However, until now, little was known about the selectivity filter gating mechanism of the channel. In this research, it was found that Ba(2+) blocked the TREK-1 channel in a concentration- and time-dependent manner. A mutagenesis analysis showed that overlapped binding of Ba(2+) at the assumed K(+) binding site 4 (S4) within the selectivity filter was responsible for the inhibitory effects on TREK-1. Then, Ba(2+) was used as a probe to explore the conformational transition in the selectivity filter of the channel. It was confirmed that collapsed conformations were induced by extracellular K(+)-free and acidification at the selectivity filters, leading to nonconductive to permeable ions. Further detailed characterization demonstrated that the two conformations presented different properties. Additionally, the N-terminal truncated isoform (ΔN41), a product derived from alternative translation initiation, was identified as a constitutively nonconductive variant. Together, these results illustrate the important role of selectivity filter gating in the regulation of TREK-1 by the extracellular K(+) and proton.
Figures


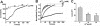
Similar articles
-
The isoforms generated by alternative translation initiation adopt similar conformation in the selectivity filter in TREK-2.J Physiol Biochem. 2015 Dec;71(4):601-10. doi: 10.1007/s13105-015-0422-z. Epub 2015 Aug 14. J Physiol Biochem. 2015. PMID: 26271386
-
The pore structure and gating mechanism of K2P channels.EMBO J. 2011 Aug 5;30(17):3607-19. doi: 10.1038/emboj.2011.268. EMBO J. 2011. PMID: 21822218 Free PMC article.
-
Polymodal Mechanism for TWIK-Related K+ Channel Inhibition by Local Anesthetic.Anesth Analg. 2019 Oct;129(4):973-982. doi: 10.1213/ANE.0000000000004216. Anesth Analg. 2019. PMID: 31124840
-
Molecular Pharmacology of K2P Potassium Channels.Cell Physiol Biochem. 2021 Mar 6;55(S3):87-107. doi: 10.33594/000000339. Cell Physiol Biochem. 2021. PMID: 33667333 Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
Ion currents, action potentials, and noradrenergic responses in rat pulmonary vein and left atrial cardiomyocytes.Physiol Rep. 2020 May;8(9):e14432. doi: 10.14814/phy2.14432. Physiol Rep. 2020. PMID: 32401431 Free PMC article.
-
Potentiation of Schaffer-Collateral CA1 Synaptic Transmission by eEF2K and p38 MAPK Mediated Mechanisms.Front Cell Neurosci. 2016 Oct 25;10:247. doi: 10.3389/fncel.2016.00247. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27826228 Free PMC article.
-
Next-generation inward rectifier potassium channel modulators: discovery and molecular pharmacology.Am J Physiol Cell Physiol. 2021 Jun 1;320(6):C1125-C1140. doi: 10.1152/ajpcell.00548.2020. Epub 2021 Apr 7. Am J Physiol Cell Physiol. 2021. PMID: 33826405 Free PMC article. Review.
-
Mutation of a single residue promotes gating of vertebrate and invertebrate two-pore domain potassium channels.Nat Commun. 2019 Feb 15;10(1):787. doi: 10.1038/s41467-019-08710-3. Nat Commun. 2019. PMID: 30770809 Free PMC article.
-
Allosteric coupling between proximal C-terminus and selectivity filter is facilitated by the movement of transmembrane segment 4 in TREK-2 channel.Sci Rep. 2016 Feb 16;6:21248. doi: 10.1038/srep21248. Sci Rep. 2016. PMID: 26879043 Free PMC article.
References
-
- Wang D. T., Hill A. P., Mann S. A., Tan P. S., Vandenberg J. I. (2011) Nat. Struct. Mol. Biol. 18, 35–41 - PubMed
-
- Domene C., Klein M. L., Branduardi D., Gervasio F. L., Parrinello M. (2008) J. Am. Chem. Soc. 130, 9474–9480 - PubMed
-
- Cordero-Morales J. F., Jogini V., Lewis A., Vásquez V., Cortes D. M., Roux B., Perozo E. (2007) Nat. Struct. Mol. Biol. 14, 1062–1069 - PubMed
-
- Cordero-Morales J. F., Cuello L. G., Zhao Y., Jogini V., Cortes D. M., Roux B., Perozo E. (2006) Nat. Struct. Mol. Biol. 13, 311–318 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

