Loss of the Drosophila cell polarity regulator Scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling
- PMID: 21955824
- PMCID: PMC3206446
- DOI: 10.1186/1471-213X-11-57
Loss of the Drosophila cell polarity regulator Scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling
Abstract
Background: Epithelial neoplasias are associated with alterations in cell polarity and excessive cell proliferation, yet how these neoplastic properties are related to one another is still poorly understood. The study of Drosophila genes that function as neoplastic tumor suppressors by regulating both of these properties has significant potential to clarify this relationship.
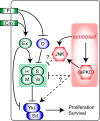
Results: Here we show in Drosophila that loss of Scribbled (Scrib), a cell polarity regulator and neoplastic tumor suppressor, results in impaired Hippo pathway signaling in the epithelial tissues of both the eye and wing imaginal disc. scrib mutant tissue overgrowth, but not the loss of cell polarity, is dependent upon defective Hippo signaling and can be rescued by knockdown of either the TEAD/TEF family transcription factor Scalloped or the transcriptional coactivator Yorkie in the eye disc, or reducing levels of Yorkie in the wing disc. Furthermore, loss of Scrib sensitizes tissue to transformation by oncogenic Ras-Raf signaling, and Yorkie-Scalloped activity is required to promote this cooperative tumor overgrowth. The inhibition of Hippo signaling in scrib mutant eye disc clones is not dependent upon JNK activity, but can be significantly rescued by reducing aPKC kinase activity, and ectopic aPKC activity is sufficient to impair Hippo signaling in the eye disc, even when JNK signaling is blocked. In contrast, warts mutant overgrowth does not require aPKC activity. Moreover, reducing endogenous levels of aPKC or increasing Scrib or Lethal giant larvae levels does not promote increased Hippo signaling, suggesting that aPKC activity is not normally rate limiting for Hippo pathway activity. Epistasis experiments suggest that Hippo pathway inhibition in scrib mutants occurs, at least in part, downstream or in parallel to both the Expanded and Fat arms of Hippo pathway regulation.
Conclusions: Loss of Scrib promotes Yorkie/Scalloped-dependent epithelial tissue overgrowth, and this is also important for driving cooperative tumor overgrowth with oncogenic Ras-Raf signaling. Whether this is also the case in human cancers now warrants investigation since the cell polarity function of Scrib and its capacity to restrain oncogene-mediated transformation, as well as the tissue growth control function of the Hippo pathway, are conserved in mammals.
Figures

Similar articles
-
Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms.Curr Biol. 2010 Apr 13;20(7):573-81. doi: 10.1016/j.cub.2010.01.055. Epub 2010 Apr 1. Curr Biol. 2010. PMID: 20362447
-
The Hippo tumor-suppressor pathway regulates apical-domain size in parallel to tissue growth.J Cell Sci. 2009 Jul 15;122(Pt 14):2351-9. doi: 10.1242/jcs.046482. Epub 2009 Jun 16. J Cell Sci. 2009. PMID: 19531584 Free PMC article.
-
Scribble mutants promote aPKC and JNK-dependent epithelial neoplasia independently of Crumbs.BMC Biol. 2009 Sep 24;7:62. doi: 10.1186/1741-7007-7-62. BMC Biol. 2009. PMID: 19778415 Free PMC article.
-
Lgl/aPKC and Crb regulate the Salvador/Warts/Hippo pathway.Fly (Austin). 2010 Oct-Dec;4(4):288-93. doi: 10.4161/fly.4.4.13116. Epub 2010 Oct 21. Fly (Austin). 2010. PMID: 20798605 Free PMC article. Review.
-
Connecting epithelial polarity, proliferation and cancer in Drosophila: the many faces of lgl loss of function.Int J Dev Biol. 2013;57(9-10):677-87. doi: 10.1387/ijdb.130285dg. Int J Dev Biol. 2013. PMID: 24395559 Review.
Cited by
-
Cooperation of the BTB-Zinc finger protein, Abrupt, with cytoskeletal regulators in Drosophila epithelial tumorigenesis.Biol Open. 2015 Jul 17;4(8):1024-39. doi: 10.1242/bio.012815. Biol Open. 2015. PMID: 26187947 Free PMC article.
-
Fear-of-intimacy-mediated zinc transport controls fat body cell dissociation through modulating Mmp activity in Drosophila.Cell Death Dis. 2021 Sep 25;12(10):874. doi: 10.1038/s41419-021-04147-z. Cell Death Dis. 2021. PMID: 34564691 Free PMC article.
-
In Drosophila, RhoGEF2 cooperates with activated Ras in tumorigenesis through a pathway involving Rho1-Rok-Myosin-II and JNK signalling.Dis Model Mech. 2013 May;6(3):661-78. doi: 10.1242/dmm.010066. Epub 2013 Jan 11. Dis Model Mech. 2013. PMID: 23324326 Free PMC article.
-
Genome-Wide Screen for Context-Dependent Tumor Suppressors Identified Using in Vivo Models for Neoplasia in Drosophila.G3 (Bethesda). 2020 Sep 2;10(9):2999-3008. doi: 10.1534/g3.120.401545. G3 (Bethesda). 2020. PMID: 32737065 Free PMC article.
-
Juvenile hormone promotes paracellular transport of yolk proteins via remodeling zonula adherens at tricellular junctions in the follicular epithelium.PLoS Genet. 2022 Jun 27;18(6):e1010292. doi: 10.1371/journal.pgen.1010292. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35759519 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

