RIG-I like receptors and their signaling crosstalk in the regulation of antiviral immunity
- PMID: 21949557
- PMCID: PMC3177754
- DOI: 10.1016/j.coviro.2011.04.004
RIG-I like receptors and their signaling crosstalk in the regulation of antiviral immunity
Abstract
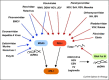
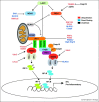
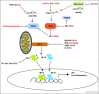
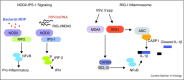
During virus infection, multiple immune signaling pathways are triggered, both within the host cell and bystander cells of an infected tissue. These pathways act in concert to mediate innate antiviral immunity and to initiate the inflammatory response against infection. The RIG-I-like receptor (RLR) family of pattern recognition receptors (PRRs) is a group of cytosolic RNA helicase proteins that can identify viral RNA as nonself via binding to pathogen associated molecular patter (PAMP) motifs within RNA ligands that accumulate during virus infection. This interaction then leads to triggering of an innate antiviral response within the infected cells through RLR induction of downstream effector molecules such as type I interferon (IFN) and other pro-inflammatory cytokines that serve to induce antiviral and inflammatory gene expression within the local tissue. Cellular regulation of RLR signaling is a critical process that can direct the outcome of infection and is essential for governance of the overall immune response and avoidance of immune toxicity. Mechanisms of positive and negative regulation of RLR signaling have been identified that include signaling crosstalk between RLR pathways and Nuclear Oligomerization Domain (NOD)-Like Receptor (NLR) pathways and Caspase networks. Furthermore, many viruses have evolved mechanisms to target these pathways to promote enhanced replication and spread within the host. These virus-host interactions therefore carry important consequences for host immunity and viral pathogenesis. Understanding the pivotal role of RLRs in immune regulation and signaling crosstalk in antiviral immunity may provide new insights into therapeutic strategies for the control of virus infection and immunity.
Keywords: RIG-I-like receptors; immunity (author to check).
Figures
Similar articles
-
[Signal transduction of innate immunity to virus infection].Bing Du Xue Bao. 2012 May;28(3):303-10. Bing Du Xue Bao. 2012. PMID: 22764536 Review. Chinese.
-
RIG-I in RNA virus recognition.Virology. 2015 May;479-480:110-21. doi: 10.1016/j.virol.2015.02.017. Epub 2015 Mar 5. Virology. 2015. PMID: 25749629 Free PMC article. Review.
-
Pattern recognition receptors and the innate immune response to viral infection.Viruses. 2011 Jun;3(6):920-40. doi: 10.3390/v3060920. Epub 2011 Jun 23. Viruses. 2011. PMID: 21994762 Free PMC article. Review.
-
Regulation of RLR-mediated innate immune signaling--it is all about keeping the balance.Eur J Cell Biol. 2012 Jan;91(1):36-47. doi: 10.1016/j.ejcb.2011.01.011. Epub 2011 Apr 9. Eur J Cell Biol. 2012. PMID: 21481967 Review.
-
Regulation of RIG-I-like receptor-mediated signaling: interaction between host and viral factors.Cell Mol Immunol. 2021 Mar;18(3):539-555. doi: 10.1038/s41423-020-00602-7. Epub 2021 Jan 18. Cell Mol Immunol. 2021. PMID: 33462384 Free PMC article. Review.
Cited by
-
Activation of host nucleic acid sensors by Mycobacterium: good for us or good for them?Crit Rev Microbiol. 2024 Mar;50(2):224-240. doi: 10.1080/1040841X.2023.2294904. Epub 2023 Dec 28. Crit Rev Microbiol. 2024. PMID: 38153209 Review.
-
Systems immunology of transcriptional responses to viral infection identifies conserved antiviral pathways across macaques and humans.Cell Rep. 2024 Feb 27;43(2):113706. doi: 10.1016/j.celrep.2024.113706. Epub 2024 Jan 30. Cell Rep. 2024. PMID: 38294906 Free PMC article.
-
Energetics of Preferential Binding of Retinoic Acid-Inducible Gene-I to Double-Stranded Viral RNAs with 5' Tri-/Diphosphate over 5' Monophosphate.ACS Omega. 2018 Apr 30;3(4):3786-3795. doi: 10.1021/acsomega.7b02019. Epub 2018 Apr 3. ACS Omega. 2018. PMID: 30023880 Free PMC article.
-
Construction of the influenza A virus infection-induced cell-specific inflammatory regulatory network based on mutual information and optimization.BMC Syst Biol. 2013 Oct 20;7:105. doi: 10.1186/1752-0509-7-105. BMC Syst Biol. 2013. PMID: 24138989 Free PMC article.
-
Type I Interferon at the Interface of Antiviral Immunity and Immune Regulation: The Curious Case of HIV-1.Scientifica (Cairo). 2013;2013:580968. doi: 10.1155/2013/580968. Epub 2013 Dec 22. Scientifica (Cairo). 2013. PMID: 24455433 Free PMC article. Review.
References
-
- Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. - PubMed
-
- Yanai H., Savitsky D., Tamura T., Taniguchi T. Regulation of the cytosolic DNA-sensing system in innate immunity: a current view. Curr Opin Immunol. 2009;21:17–22. - PubMed
-
- Saito T., Gale M., Jr. Principles of intracellular viral recognition. Curr Opin Immunol. 2007;19:17–23. - PubMed
-
- Hiscott J., Lin R., Nakhaei P., Paz S. MasterCARD: a priceless link to innate immunity. Trends Mol Med. 2006;12:53–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

