Aquaporin-4 Reduces Post-Traumatic Seizure Susceptibility by Promoting Astrocytic Glial Scar Formation in Mice
- PMID: 21939392
- PMCID: PMC8418529
- DOI: 10.1089/neu.2011.2114
Aquaporin-4 Reduces Post-Traumatic Seizure Susceptibility by Promoting Astrocytic Glial Scar Formation in Mice
Abstract
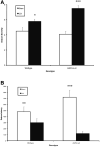
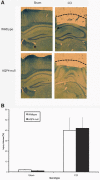
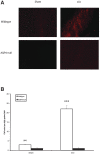
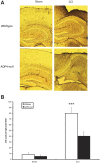
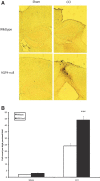
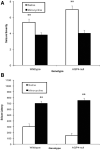
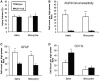
Seizures are important neurological complications after traumatic brain injury (TBI) and are reported for up to 50% of patients with TBI. Despite several studies, no drug strategy has been able to alter the biological events leading to epileptogenesis. The glial water channel, aquaporin-4 (AQP4), was shown to facilitate cytotoxic cell swelling in ischemia and glial scar formation after stab wound injury. In this study, we examined post-traumatic seizure susceptibility of AQP4-deficient mice (AQP4-/-) after injection of pentylenetetrazole (PTZ) 1 month after controlled cortical impact (CCI) and compared them to wild-type sham injury controls. After PTZ injection, AQP4-/- mice demonstrated dramatically shortened seizure latency (120 ± 40 vs. 300 ± 70 sec; p < 0.001) and increased seizure severity (grade 7.5 ± 0.4 vs. 5.8 ± 0.4; p < 0.001) compared to their wild-type counterparts. Morphometric analysis demonstrated a significant 2-fold reduction in astrocytosis, with a concomitant increase in microgliosis in injured AQP4-null mice compared to their injured wild-type counterparts (44 ± 2 vs. 24 ± 3 cells per high power field [cells/hpf], respectively; p < 0.0001). Minocycline, an inhibitor of microglia, reversed the post-TBI epilepsy phenotype of AQP4-null mice. After minocycline treatment, AQP4-/- mice demonstrated similar latency of seizures evoked by PTZ (723 ± 35 vs. 696 ± 38 sec; p > 0.05) and severity of seizures evoked by PTZ (grade 4.0 ± 0.5 vs. 3.81 ± 0.30; p > 0.05) compared to wild-type counterparts. Immunohistochemical analysis demonstrated decreased immunostaining of microglia to levels comparable to wild-type (12 ± 2 vs. 11 ± 4 cells/hpf, respectively; p > 0.05). Taken together, these results suggest a protective role of AQP4 in post-traumatic seizure susceptibility by promoting astrogliosis, formation of a glial scar, and preventing microgliosis.
Keywords: aquaporin; astrocyte; glial scar; seizure epilepsy.
Conflict of interest statement
No competing financial interests exist.
Figures

Similar articles
-
Modulation of posttraumatic epileptogenesis in aquaporin-4 knockout mice.Epilepsia. 2020 Jul;61(7):1503-1514. doi: 10.1111/epi.16551. Epub 2020 Jun 2. Epilepsia. 2020. PMID: 32484924
-
Inhibitory role of lentivirus-mediated aquaporin-4 gene silencing in the formation of glial scar in a rat model of traumatic brain injury.J Cell Biochem. 2019 Jan;120(1):368-379. doi: 10.1002/jcb.27390. Epub 2018 Sep 23. J Cell Biochem. 2019. Retraction in: J Cell Biochem. 2021 Nov;122 Suppl 1:S118. doi: 10.1002/jcb.30098. PMID: 30246455 Retracted.
-
Aquaporin-4 mitigates retrograde degeneration of rubrospinal neurons by facilitating edema clearance and glial scar formation after spinal cord injury in mice.Mol Neurobiol. 2014 Jun;49(3):1327-37. doi: 10.1007/s12035-013-8607-3. Epub 2014 Jan 4. Mol Neurobiol. 2014. PMID: 24390474
-
Three distinct roles of aquaporin-4 in brain function revealed by knockout mice.Biochim Biophys Acta. 2006 Aug;1758(8):1085-93. doi: 10.1016/j.bbamem.2006.02.018. Epub 2006 Mar 10. Biochim Biophys Acta. 2006. PMID: 16564496 Review.
-
New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice.Neuroscience. 2004;129(4):983-91. doi: 10.1016/j.neuroscience.2004.06.088. Neuroscience. 2004. PMID: 15561413 Review.
Cited by
-
Different aquaporin-4 expression in glioblastoma multiforme patients with and without seizures.Mol Med. 2012 Sep 25;18(1):1147-51. doi: 10.2119/molmed.2012.00015. Mol Med. 2012. PMID: 22714714 Free PMC article.
-
Novel frontiers in epilepsy treatments: preventing epileptogenesis by targeting inflammation.Expert Rev Neurother. 2013 Jun;13(6):615-25. doi: 10.1586/ern.13.54. Expert Rev Neurother. 2013. PMID: 23738999 Free PMC article. Review.
-
Aquaporin and brain diseases.Biochim Biophys Acta. 2014 May;1840(5):1554-65. doi: 10.1016/j.bbagen.2013.10.032. Epub 2013 Oct 26. Biochim Biophys Acta. 2014. PMID: 24513456 Free PMC article. Review.
-
The Role of Aquaporins in Spinal Cord Injury.Cells. 2023 Jun 23;12(13):1701. doi: 10.3390/cells12131701. Cells. 2023. PMID: 37443735 Free PMC article. Review.
-
Aquaporin 4: a player in cerebral edema and neuroinflammation.J Neuroinflammation. 2012 Dec 27;9:279. doi: 10.1186/1742-2094-9-279. J Neuroinflammation. 2012. PMID: 23270503 Free PMC article. Review.
References
-
- Annegers, J.F., and Coan, S.P. (2000). The risks of epilepsy after traumatic brain injury. Seizure 9, 453–457 - PubMed
-
- Jennett, B., Teather, D., and Bennie, S. (1973). Epilepsy after head injury. Residual risk after varying fit-free intervals since injury. Lancet 2, 652–653 - PubMed
-
- Semah, F., Picot, M.C., Adam, C., Broglin, D., Arzimanoglou, A., Bazin, B., Cavalcanti, D., and Baulac, M. (1998). Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology 51, 1256–1262 - PubMed
-
- Iudice, A., and Murri, L. (2000). Pharmacological prophylaxis of post-traumatic epilepsy. Drugs 59, 1091–1099 - PubMed
-
- Teasell, R., Bayona, N., Lippert, C., Villamere, J., and Hellings, C. (2007). Post-traumatic seizure disorder following acquired brain injury. Brain Inj. 21, 201–214 - PubMed

