Cryo-electron tomography reveals conserved features of doublet microtubules in flagella
- PMID: 21930914
- PMCID: PMC3198354
- DOI: 10.1073/pnas.1106178108
Cryo-electron tomography reveals conserved features of doublet microtubules in flagella
Abstract
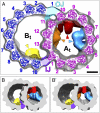
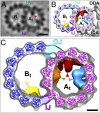
The axoneme forms the essential and conserved core of cilia and flagella. We have used cryo-electron tomography of Chlamydomonas and sea urchin flagella to answer long-standing questions and to provide information about the structure of axonemal doublet microtubules (DMTs). Solving an ongoing controversy, we show that B-tubules of DMTs contain exactly 10 protofilaments (PFs) and that the inner junction (IJ) and outer junction between the A- and B-tubules are fundamentally different. The outer junction, crucial for the initiation of doublet formation, appears to be formed by close interactions between the tubulin subunits of three PFs with unusual tubulin interfaces; other investigators have reported that this junction is weakened by mutations affecting posttranslational modifications of tubulin. The IJ consists of an axially periodic ladder-like structure connecting tubulin PFs of the A- and B-tubules. The recently discovered microtubule inner proteins (MIPs) on the inside of the A- and B-tubules are more complex than previously thought. They are composed of alternating small and large subunits with periodicities of 16 and/or 48 nm. MIP3 forms arches connecting B-tubule PFs, contrary to an earlier report that MIP3 forms the IJ. Finally, the "beak" structures within the B-tubules of Chlamydomonas DMT1, DMT5, and DMT6 are clearly composed of a longitudinal band of proteins repeating with a periodicity of 16 nm. These findings, discussed in relation to genetic and biochemical data, provide a critical foundation for future work on the molecular assembly and stability of the axoneme, as well as its function in motility and sensory transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Insights into the structure and function of ciliary and flagellar doublet microtubules: tektins, Ca2+-binding proteins, and stable protofilaments.J Biol Chem. 2014 Jun 20;289(25):17427-44. doi: 10.1074/jbc.M114.568949. Epub 2014 May 2. J Biol Chem. 2014. PMID: 24794867 Free PMC article.
-
FAP20 is an inner junction protein of doublet microtubules essential for both the planar asymmetrical waveform and stability of flagella in Chlamydomonas.Mol Biol Cell. 2014 May;25(9):1472-83. doi: 10.1091/mbc.E13-08-0464. Epub 2014 Feb 26. Mol Biol Cell. 2014. PMID: 24574454 Free PMC article.
-
At least one of the protofilaments in flagellar microtubules is not composed of tubulin.Curr Biol. 1995 Feb 1;5(2):158-67. doi: 10.1016/s0960-9822(95)00037-6. Curr Biol. 1995. PMID: 7743179
-
Microtubule Inner Proteins: A Meshwork of Luminal Proteins Stabilizing the Doublet Microtubule.Bioessays. 2018 Mar;40(3). doi: 10.1002/bies.201700209. Epub 2018 Feb 12. Bioessays. 2018. PMID: 29430673 Review.
-
Three-dimensional structural labeling microscopy of cilia and flagella.Microscopy (Oxf). 2017 Aug 1;66(4):234-244. doi: 10.1093/jmicro/dfx018. Microscopy (Oxf). 2017. PMID: 28541401 Review.
Cited by
-
Cryoelectron tomography reveals doublet-specific structures and unique interactions in the I1 dynein.Proc Natl Acad Sci U S A. 2012 Jul 24;109(30):E2067-76. doi: 10.1073/pnas.1120690109. Epub 2012 Jun 25. Proc Natl Acad Sci U S A. 2012. PMID: 22733763 Free PMC article.
-
A detailed, hierarchical study of Giardia lamblia's ventral disc reveals novel microtubule-associated protein complexes.PLoS One. 2012;7(9):e43783. doi: 10.1371/journal.pone.0043783. Epub 2012 Sep 11. PLoS One. 2012. PMID: 22984443 Free PMC article.
-
FAP20 is required for flagellum assembly in Trypanosoma brucei.bioRxiv [Preprint]. 2024 Jan 20:2024.01.19.576295. doi: 10.1101/2024.01.19.576295. bioRxiv. 2024. Update in: Mol Biol Cell. 2024 Nov 1;35(11):br22. doi: 10.1091/mbc.E23-12-0497. PMID: 38293126 Free PMC article. Updated. Preprint.
-
Tubulin-dynein system in flagellar and ciliary movement.Proc Jpn Acad Ser B Phys Biol Sci. 2012;88(8):397-415. doi: 10.2183/pjab.88.397. Proc Jpn Acad Ser B Phys Biol Sci. 2012. PMID: 23060230 Free PMC article. Review.
-
Structural and molecular bases of rod photoreceptor morphogenesis and disease.Prog Retin Eye Res. 2016 Nov;55:32-51. doi: 10.1016/j.preteyeres.2016.06.002. Epub 2016 Jun 22. Prog Retin Eye Res. 2016. PMID: 27352937 Free PMC article. Review.
References
-
- Fliegauf M, Benzing T, Omran H. When cilia go bad: Cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. - PubMed
-
- Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int J Syst Evol Microbiol. 2002;52:297–354. - PubMed
-
- Kubo T, Yanagisawa HA, Yagi T, Hirono M, Kamiya R. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr Biol. 2010;20:441–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

