Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation
- PMID: 21930768
- PMCID: PMC3182052
- DOI: 10.1084/jem.20110522
Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation
Abstract
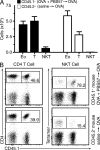
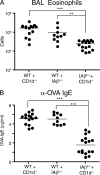
Airborne exposure to microbial cell wall lipids such as lipopolysaccharide triggers innate immune responses that regulate susceptibility to allergic airway inflammation. α-Glycosylceramides represent another widespread class of microbial lipids that directly stimulate innate-like, IL-4- and IL-13-producing, CD1d-restricted NKT cells. In this study, we demonstrate that NKT cells constitutively accumulate and reside in the microvasculature of the mouse lung. After a single airborne exposure to lipid antigen, they promptly extravasate to orchestrate the formation of peribronchiolar and interstitial lymphohistiocytic granulomas containing numerous eosinophils. Concomitant airborne exposure to ovalbumin (OVA) induces the priming of OVA-specific Th2 cells and IgE antibodies by the same dendritic cell coexpressing CD1d and MHC class II. Although NKT cell activation remains confined to the lipid-exposed lung and draining lymph nodes, Th2 cells recirculate and seed the lung of a parabiotic partner, conferring susceptibility to OVA challenge months after the initial exposure, in a manner independent of NKT cells and CD1d. Thus, transient recruitment and activation of lung-resident intravascular NKT cells can trigger long-term susceptibility to allergic airway inflammation.
Figures



Similar articles
-
Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model.J Immunol. 2003 Aug 15;171(4):1637-41. doi: 10.4049/jimmunol.171.4.1637. J Immunol. 2003. PMID: 12902459
-
Antigen-Specific IgG ameliorates allergic airway inflammation via Fcγ receptor IIB on dendritic cells.Respir Res. 2011 Apr 10;12(1):42. doi: 10.1186/1465-9921-12-42. Respir Res. 2011. PMID: 21477339 Free PMC article.
-
α-Galactosylceramide-induced airway eosinophilia is mediated through the activation of NKT cells.J Immunol. 2011 Apr 15;186(8):4687-92. doi: 10.4049/jimmunol.1003659. Epub 2011 Mar 7. J Immunol. 2011. PMID: 21383248
-
Natural killer T cells are not the predominant T cell in asthma and likely modulate, not cause, asthma.J Allergy Clin Immunol. 2010 May;125(5):980-4. doi: 10.1016/j.jaci.2010.01.032. Epub 2010 Mar 20. J Allergy Clin Immunol. 2010. PMID: 20304475 Free PMC article. Review.
-
Antigen recognition by CD1d-restricted NKT T cell receptors.Semin Immunol. 2010 Apr;22(2):61-7. doi: 10.1016/j.smim.2009.10.004. Epub 2009 Nov 28. Semin Immunol. 2010. PMID: 19945889 Review.
Cited by
-
Intestinal mucus-derived nanoparticle-mediated activation of Wnt/β-catenin signaling plays a role in induction of liver natural killer T cell anergy in mice.Hepatology. 2013 Mar;57(3):1250-61. doi: 10.1002/hep.26086. Epub 2013 Feb 11. Hepatology. 2013. PMID: 22991247 Free PMC article.
-
The Role of Lipids in Development of Allergic Responses.Immune Netw. 2017 Jun;17(3):133-143. doi: 10.4110/in.2017.17.3.133. Epub 2017 Jun 20. Immune Netw. 2017. PMID: 28680374 Free PMC article. Review.
-
Emerging features of MAIT cells and other unconventional T cell populations in human viral disease and vaccination.Semin Immunol. 2022 Nov;61-64:101661. doi: 10.1016/j.smim.2022.101661. Epub 2022 Oct 28. Semin Immunol. 2022. PMID: 36374780 Free PMC article. Review.
-
Generation and characterization of CD1d-specific single-domain antibodies with distinct functional features.Immunology. 2016 Sep;149(1):111-21. doi: 10.1111/imm.12635. Immunology. 2016. PMID: 27312006 Free PMC article.
-
Invariant Natural Killer T Cell Subsets-More Than Just Developmental Intermediates.Front Immunol. 2018 Jun 20;9:1393. doi: 10.3389/fimmu.2018.01393. eCollection 2018. Front Immunol. 2018. PMID: 29973936 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

