Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels
- PMID: 21926172
- PMCID: PMC3234757
- DOI: 10.1074/jbc.M111.264341
Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels
Abstract
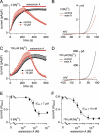
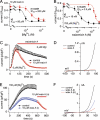
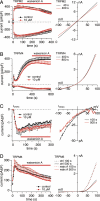
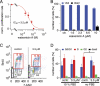
Transient receptor potential melastatin 7 (TRPM7) channels represent the major magnesium-uptake mechanism in mammalian cells and are key regulators of cell growth and proliferation. They are expressed abundantly in a variety of human carcinoma cells controlling survival, growth, and migration. These characteristics are the basis for recent interest in the channel as a target for cancer therapeutics. We screened a chemical library of marine organism-derived extracts and identified waixenicin A from the soft coral Sarcothelia edmondsoni as a strong inhibitor of overexpressed and native TRPM7. Waixenicin A activity was cytosolic and potentiated by intracellular free magnesium (Mg(2+)) concentration. Mutating a Mg(2+) binding site on the TRPM7 kinase domain reduced the potency of the compound, whereas kinase deletion enhanced its efficacy independent of Mg(2+). Waixenicin A failed to inhibit the closely homologous TRPM6 channel and did not significantly affect TRPM2, TRPM4, and Ca(2+) release-activated Ca(2+) current channels. Therefore, waixenicin A represents the first potent and relatively specific inhibitor of TRPM7 ion channels. Consistent with TRPM7 inhibition, the compound blocked cell proliferation in human Jurkat T-cells and rat basophilic leukemia cells. Based on the ability of the compound to inhibit cell proliferation through Mg(2+)-dependent block of TRPM7, waixenicin A, or structural analogs may have cancer-specific therapeutic potential, particularly because certain cancers accumulate cytosolic Mg(2+).
Figures

Similar articles
-
Waixenicin A, a marine-derived TRPM7 inhibitor: a promising CNS drug lead.Acta Pharmacol Sin. 2020 Dec;41(12):1519-1524. doi: 10.1038/s41401-020-00512-4. Epub 2020 Sep 29. Acta Pharmacol Sin. 2020. PMID: 32994545 Free PMC article. Review.
-
Inhibition of TRPM7 with waixenicin A reduces glioblastoma cellular functions.Cell Calcium. 2020 Dec;92:102307. doi: 10.1016/j.ceca.2020.102307. Epub 2020 Oct 14. Cell Calcium. 2020. PMID: 33080445
-
Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7.Br J Pharmacol. 2012 Jun;166(4):1357-76. doi: 10.1111/j.1476-5381.2012.01855.x. Br J Pharmacol. 2012. PMID: 22242975 Free PMC article.
-
TRPM6 kinase activity regulates TRPM7 trafficking and inhibits cellular growth under hypomagnesic conditions.Cell Mol Life Sci. 2014 Dec;71(24):4853-67. doi: 10.1007/s00018-014-1647-7. Epub 2014 May 25. Cell Mol Life Sci. 2014. PMID: 24858416 Free PMC article.
-
Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: implications in hypertension.Am J Physiol Heart Circ Physiol. 2008 Mar;294(3):H1103-18. doi: 10.1152/ajpheart.00903.2007. Epub 2008 Jan 11. Am J Physiol Heart Circ Physiol. 2008. PMID: 18192217 Review.
Cited by
-
The Role of TRPM7 in Oncogenesis.Int J Mol Sci. 2024 Jan 5;25(2):719. doi: 10.3390/ijms25020719. Int J Mol Sci. 2024. PMID: 38255793 Free PMC article. Review.
-
pH-Channeling in Cancer: How pH-Dependence of Cation Channels Shapes Cancer Pathophysiology.Cancers (Basel). 2020 Sep 2;12(9):2484. doi: 10.3390/cancers12092484. Cancers (Basel). 2020. PMID: 32887220 Free PMC article. Review.
-
TRPM7 Ion Channel: Oncogenic Roles and Therapeutic Potential in Breast Cancer.Cancers (Basel). 2021 Dec 16;13(24):6322. doi: 10.3390/cancers13246322. Cancers (Basel). 2021. PMID: 34944940 Free PMC article. Review.
-
The Pathophysiologic Roles of TRPM7 Channel.Korean J Physiol Pharmacol. 2014 Feb;18(1):15-23. doi: 10.4196/kjpp.2014.18.1.15. Epub 2014 Feb 13. Korean J Physiol Pharmacol. 2014. PMID: 24634592 Free PMC article. Review.
-
TRPM7.Handb Exp Pharmacol. 2014;222:521-46. doi: 10.1007/978-3-642-54215-2_21. Handb Exp Pharmacol. 2014. PMID: 24756720 Free PMC article. Review.
References
-
- Nadler M. J., Hermosura M. C., Inabe K., Perraud A. L., Zhu Q., Stokes A. J., Kurosaki T., Kinet J. P., Penner R., Scharenberg A. M., Fleig A. (2001) Nature 411, 590–595 - PubMed
-
- Ryazanova L. V., Dorovkov M. V., Ansari A., Ryazanov A. G. (2004) J. Biol. Chem. 279, 3708–3716 - PubMed
-
- Chubanov V., Gudermann T., Schlingmann K. P. (2005) Pflugers Arch. 451, 228–234 - PubMed
-
- Voets T., Nilius B., Hoefs S., van der Kemp A. W., Droogmans G., Bindels R. J., Hoenderop J. G. (2004) J. Biol. Chem. 279, 19–25 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

