Protein kinase PKR and RNA adenosine deaminase ADAR1: new roles for old players as modulators of the interferon response
- PMID: 21924887
- PMCID: PMC3190076
- DOI: 10.1016/j.coi.2011.08.009
Protein kinase PKR and RNA adenosine deaminase ADAR1: new roles for old players as modulators of the interferon response
Abstract
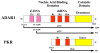
Double-stranded RNA (dsRNA) plays a centrally important role in antiviral innate immunity, both for the production of interferon (IFN) and also in the actions of IFN. Among the IFN-inducible gene products are the protein kinase regulated by RNA (PKR) and the adenosine deaminase acting on RNA 1 (ADAR1). PKR is an established key player in the antiviral actions of IFN, through dsRNA-dependent activation and subsequent phosphorylation of protein synthesis initiation factor eIF2α thereby altering the translational pattern in cells. In addition, PKR plays an important role as a positive effector that amplifies the production of IFN. ADAR1 catalyzes the deamination of adenosine (A) in RNA with double-stranded (ds) character, leading to the destabilization of RNA duplex structures and genetic recoding. By contrast to the antiviral and proapoptotic functions associated with PKR, the actions of ADAR1 in some instances are proviral and cell protective as ADAR1 functions as a suppressor of dsRNA-mediated antiviral responses including activation of PKR and interferon regulatory factor 3.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
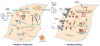


Similar articles
-
Tipping the balance: antagonism of PKR kinase and ADAR1 deaminase functions by virus gene products.J Interferon Cytokine Res. 2009 Sep;29(9):477-87. doi: 10.1089/jir.2009.0065. J Interferon Cytokine Res. 2009. PMID: 19715457 Free PMC article. Review.
-
Editing of Cellular Self-RNAs by Adenosine Deaminase ADAR1 Suppresses Innate Immune Stress Responses.J Biol Chem. 2016 Mar 18;291(12):6158-68. doi: 10.1074/jbc.M115.709014. Epub 2016 Jan 27. J Biol Chem. 2016. PMID: 26817845 Free PMC article.
-
RNA-specific adenosine deaminase ADAR1 suppresses measles virus-induced apoptosis and activation of protein kinase PKR.J Biol Chem. 2009 Oct 23;284(43):29350-6. doi: 10.1074/jbc.M109.045146. Epub 2009 Aug 25. J Biol Chem. 2009. PMID: 19710021 Free PMC article.
-
Adenosine deaminase ADAR1 increases gene expression at the translational level by decreasing protein kinase PKR-dependent eIF-2alpha phosphorylation.J Mol Biol. 2009 Nov 6;393(4):777-87. doi: 10.1016/j.jmb.2009.08.070. Epub 2009 Sep 3. J Mol Biol. 2009. PMID: 19733181 Free PMC article.
-
Interferon action and the double-stranded RNA-dependent enzymes ADAR1 adenosine deaminase and PKR protein kinase.Prog Nucleic Acid Res Mol Biol. 2006;81:369-434. doi: 10.1016/S0079-6603(06)81010-X. Prog Nucleic Acid Res Mol Biol. 2006. PMID: 16891177 Review. No abstract available.
Cited by
-
Dissemination of Piscine orthoreovirus-1 (PRV-1) in Atlantic Salmon (Salmo salar) during the Early and Regenerating Phases of Infection.Pathogens. 2020 Feb 20;9(2):143. doi: 10.3390/pathogens9020143. Pathogens. 2020. PMID: 32093243 Free PMC article.
-
Active Ebola Virus Replication and Heterogeneous Evolutionary Rates in EVD Survivors.Cell Rep. 2018 Jan 30;22(5):1159-1168. doi: 10.1016/j.celrep.2018.01.008. Cell Rep. 2018. PMID: 29386105 Free PMC article.
-
Shared and Distinct Functions of Type I and Type III Interferons.Immunity. 2019 Apr 16;50(4):907-923. doi: 10.1016/j.immuni.2019.03.025. Immunity. 2019. PMID: 30995506 Free PMC article. Review.
-
Cytoplasmic sensing of viral nucleic acids.Curr Opin Virol. 2015 Apr;11:31-7. doi: 10.1016/j.coviro.2015.01.012. Epub 2015 Feb 7. Curr Opin Virol. 2015. PMID: 25668758 Free PMC article. Review.
-
Intracellular detection of viral nucleic acids.Curr Opin Microbiol. 2015 Aug;26:1-9. doi: 10.1016/j.mib.2015.03.001. Epub 2015 Mar 18. Curr Opin Microbiol. 2015. PMID: 25795286 Free PMC article. Review.
References
-
- Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. - PubMed
-
- Colby C, Morgan MJ. Interferon induction and action. Annu Rev Microbiol. 1971;25:333–360. - PubMed
-
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

