Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3
- PMID: 21921027
- PMCID: PMC3207424
- DOI: 10.1074/jbc.M111.268938
Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3
Abstract
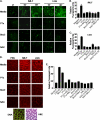
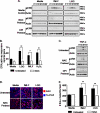
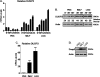
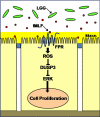
The normal microbial occupants of the mammalian intestine are crucial for maintaining gut homeostasis, yet the mechanisms by which intestinal cells perceive and respond to the microbiota are largely unknown. Intestinal epithelial contact with commensal bacteria and/or their products has been shown to activate noninflammatory signaling pathways, such as extracellular signal-related kinase (ERK), thus influencing homeostatic processes. We previously demonstrated that commensal bacteria stimulate ERK pathway activity via interaction with formyl peptide receptors (FPRs). In the current study, we expand on these findings and show that commensal bacteria initiate ERK signaling through rapid FPR-dependent reactive oxygen species (ROS) generation and subsequent modulation of MAP kinase phosphatase redox status. ROS generation induced by the commensal bacteria Lactobacillus rhamnosus GG and the FPR peptide ligand, N-formyl-Met-Leu-Phe, was abolished in the presence of selective inhibitors for G protein-coupled signaling and FPR ligand interaction. In addition, pretreatment of cells with inhibitors of ROS generation attenuated commensal bacteria-induced ERK signaling, indicating that ROS generation is required for ERK pathway activation. Bacterial colonization also led to oxidative inactivation of the redox-sensitive and ERK-specific phosphatase, DUSP3/VHR, and consequent stimulation of ERK pathway signaling. Together, these data demonstrate that commensal bacteria and their products activate ROS signaling in an FPR-dependent manner and define a mechanism by which cellular ROS influences the ERK pathway through a redox-sensitive regulatory circuit.
Figures

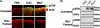
Similar articles
-
Commensal-epithelial signaling mediated via formyl peptide receptors.Am J Pathol. 2010 Dec;177(6):2782-90. doi: 10.2353/ajpath.2010.100529. Epub 2010 Oct 29. Am J Pathol. 2010. PMID: 21037077 Free PMC article.
-
Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1.Mucosal Immunol. 2014 May;7(3):645-55. doi: 10.1038/mi.2013.84. Epub 2013 Nov 6. Mucosal Immunol. 2014. PMID: 24192910 Free PMC article.
-
Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications.Curr Med Chem. 2012;19(10):1519-29. doi: 10.2174/092986712799828283. Curr Med Chem. 2012. PMID: 22360484 Free PMC article. Review.
-
Epithelial adhesion mediated by pilin SpaC is required for Lactobacillus rhamnosus GG-induced cellular responses.Appl Environ Microbiol. 2014 Aug;80(16):5068-77. doi: 10.1128/AEM.01039-14. Epub 2014 Jun 13. Appl Environ Microbiol. 2014. PMID: 24928883 Free PMC article.
-
Redox signaling mediated by the gut microbiota.Free Radic Biol Med. 2017 Apr;105:41-47. doi: 10.1016/j.freeradbiomed.2016.10.495. Epub 2016 Oct 29. Free Radic Biol Med. 2017. PMID: 27989756 Review.
Cited by
-
Annexin A1: shifting the balance towards resolution and repair.Biol Chem. 2016 Oct 1;397(10):971-9. doi: 10.1515/hsz-2016-0180. Biol Chem. 2016. PMID: 27232634 Free PMC article. Review.
-
GPCR transactivation signalling in vascular smooth muscle cells: role of NADPH oxidases and reactive oxygen species.Vasc Biol. 2019 Jul 23;1(1):R1-R11. doi: 10.1530/VB-18-0004. eCollection 2019. Vasc Biol. 2019. PMID: 32923966 Free PMC article. Review.
-
Lactobacillus rhamnosus GG-induced Expression of Leptin in the Intestine Orchestrates Epithelial Cell Proliferation.Cell Mol Gastroenterol Hepatol. 2020;9(4):627-639. doi: 10.1016/j.jcmgh.2019.12.004. Epub 2019 Dec 23. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31874255 Free PMC article.
-
Probiotics in Intestinal Mucosal Healing: A New Therapy or an Old Friend?Pharmaceuticals (Basel). 2021 Nov 19;14(11):1181. doi: 10.3390/ph14111181. Pharmaceuticals (Basel). 2021. PMID: 34832962 Free PMC article. Review.
-
Alternative use of Bacillus subtilis spores: protection against environmental oxidative stress in human normal keratinocytes.Sci Rep. 2018 Jan 29;8(1):1745. doi: 10.1038/s41598-018-20153-2. Sci Rep. 2018. PMID: 29379084 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

