Membrane topological structure of neutral system N/A amino acid transporter 4 (SNAT4) protein
- PMID: 21917917
- PMCID: PMC3207422
- DOI: 10.1074/jbc.M111.220277
Membrane topological structure of neutral system N/A amino acid transporter 4 (SNAT4) protein
Abstract
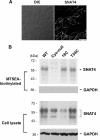
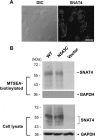
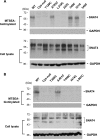
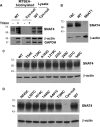
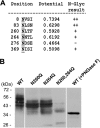
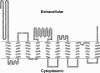
Members of system N/A amino acid transporter (SNAT) family mediate transport of neutral amino acids, including l-alanine, l-glutamine, and l-histidine, across the plasma membrane and are involved in a variety of cellular functions. By using chemical labeling, glycosylation, immunofluorescence combined with molecular modeling approaches, we resolved the membrane topological structure of SNAT4, a transporter expressed predominantly in liver. To analyze the orientation using the chemical labeling and biotinylation approach, the "Cys-null" mutant of SNAT4 was first generated by mutating all five endogenous cysteine residues. Based on predicted topological structures, a single cysteine residue was introduced individually into all possible nontransmembrane domains of the Cys-null mutant. The cells expressing these mutants were labeled with N-biotinylaminoethyl methanethiosulfonate, a membrane-impermeable cysteine-directed reagent. We mapped the orientations of N- and C-terminal domains. There are three extracellular loop domains, and among them, the second loop domain is the largest that spans from amino acid residue ∼242 to ∼335. The orientation of this domain was further confirmed by the identification of two N-glycosylated residues, Asn-260 and Asn-264. Together, we showed that SNAT4 contains 10 transmembrane domains with extracellular N and C termini and a large N-glycosylated, extracellular loop domain. This is the first report concerning membrane topological structure of mammalian SNAT transporters, which will provide important implications for our understanding of structure-function of the members in this amino acid transporter family.
Figures

Similar articles
-
Identification of a disulfide bridge important for transport function of SNAT4 neutral amino acid transporter.PLoS One. 2013;8(2):e56792. doi: 10.1371/journal.pone.0056792. Epub 2013 Feb 22. PLoS One. 2013. PMID: 23451088 Free PMC article.
-
N-Glycosylation influences transport, but not cellular trafficking, of a neuronal amino acid transporter SNAT1.Biochem J. 2016 Nov 15;473(22):4227-4242. doi: 10.1042/BCJ20160724. Epub 2016 Sep 21. Biochem J. 2016. PMID: 27655909
-
Residue cysteine 232 is important for substrate transport of neutral amino acid transporter, SNAT4.Int J Biochem Mol Biol. 2012;3(4):374-83. Epub 2012 Dec 24. Int J Biochem Mol Biol. 2012. PMID: 23301202 Free PMC article.
-
From membrane to molecule to the third amino acid from the left with a membrane transport protein.Q Rev Biophys. 1997 Nov;30(4):333-64. doi: 10.1017/s0033583597003387. Q Rev Biophys. 1997. PMID: 9634651 Review.
-
Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family.Pflugers Arch. 2004 Feb;447(5):784-95. doi: 10.1007/s00424-003-1117-9. Epub 2003 Jul 4. Pflugers Arch. 2004. PMID: 12845534 Review.
Cited by
-
The SLC38 family of sodium-amino acid co-transporters.Pflugers Arch. 2014 Jan;466(1):155-72. doi: 10.1007/s00424-013-1393-y. Epub 2013 Nov 6. Pflugers Arch. 2014. PMID: 24193407 Review.
-
Identification of a disulfide bridge important for transport function of SNAT4 neutral amino acid transporter.PLoS One. 2013;8(2):e56792. doi: 10.1371/journal.pone.0056792. Epub 2013 Feb 22. PLoS One. 2013. PMID: 23451088 Free PMC article.
-
SLC38A4 functions as a tumour suppressor in hepatocellular carcinoma through modulating Wnt/β-catenin/MYC/HMGCS2 axis.Br J Cancer. 2021 Sep;125(6):865-876. doi: 10.1038/s41416-021-01490-y. Epub 2021 Jul 17. Br J Cancer. 2021. PMID: 34274945 Free PMC article.
-
Determining expression changes of ANO7 and SLC38A4 membrane transporters in colorectal cancer.Heliyon. 2024 Jul 11;10(14):e34464. doi: 10.1016/j.heliyon.2024.e34464. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39114022 Free PMC article.
-
Membrane transporters for the special amino acid glutamine: structure/function relationships and relevance to human health.Front Chem. 2014 Aug 11;2:61. doi: 10.3389/fchem.2014.00061. eCollection 2014. Front Chem. 2014. PMID: 25157349 Free PMC article. Review.
References
-
- Christensen H. N. (1990) Physiol. Rev. 70, 43–77 - PubMed
-
- Palacín M., Estévez R., Bertran J., Zorzano A. (1998) Physiol. Rev. 78, 969–1054 - PubMed
-
- Castagna M., Shayakul C., Trotti D., Sacchi V. F., Harvey W. R., Hediger M. A. (1997) J. Exp. Biol. 200, 269–286 - PubMed
-
- Malandro M. S., Kilberg M. S. (1996) Annu. Rev. Biochem. 65, 305–336 - PubMed
-
- Mackenzie B., Erickson J. D. (2004) Pflügers Arch. 447, 784–795 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

