Phosphorylation of the kinase interaction motif in mitogen-activated protein (MAP) kinase phosphatase-4 mediates cross-talk between protein kinase A and MAP kinase signaling pathways
- PMID: 21908610
- PMCID: PMC3207433
- DOI: 10.1074/jbc.M111.255844
Phosphorylation of the kinase interaction motif in mitogen-activated protein (MAP) kinase phosphatase-4 mediates cross-talk between protein kinase A and MAP kinase signaling pathways
Abstract
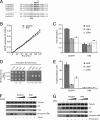
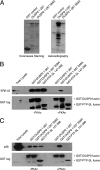
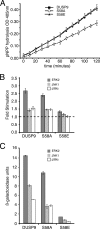
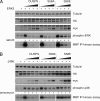
MAP kinase phosphatase 4 (DUSP9/MKP-4) plays an essential role during placental development and is one of a subfamily of three closely related cytoplasmic dual-specificity MAPK phosphatases, which includes the ERK-specific enzymes DUSP6/MKP-3 and DUSP7/MKP-X. However, unlike DUSP6/MKP-3, DUSP9/MKP-4 also inactivates the p38α MAP kinase both in vitro and in vivo. Here we demonstrate that inactivation of both ERK1/2 and p38α by DUSP9/MKP-4 is mediated by a conserved arginine-rich kinase interaction motif located within the amino-terminal non-catalytic domain of the protein. Furthermore, DUSP9/MKP-4 is unique among these cytoplasmic MKPs in containing a conserved PKA consensus phosphorylation site (55)RRXSer-58 immediately adjacent to the kinase interaction motif. DUSP9/MKP-4 is phosphorylated on Ser-58 by PKA in vitro, and phosphorylation abrogates the binding of DUSP9/MKP-4 to both ERK2 and p38α MAP kinases. In addition, although mutation of Ser-58 to either alanine or glutamic acid does not affect the intrinsic catalytic activity of DUSP9/MKP-4, phospho-mimetic (Ser-58 to Glu) substitution inhibits both the interaction of DUSP9/MKP-4 with ERK2 and p38α in vivo and its ability to dephosphorylate and inactivate these MAP kinases. Finally, the use of a phospho-specific antibody demonstrates that endogenous DUSP9/MKP-4 is phosphorylated on Ser-58 in response to the PKA agonist forskolin and is also modified in placental tissue. We conclude that DUSP9/MKP-4 is a bona fide target of PKA signaling and that attenuation of DUSP9/MKP-4 function can mediate cross-talk between the PKA pathway and MAPK signaling through both ERK1/2 and p38α in vivo.
Figures

Similar articles
-
Distinct binding determinants for ERK2/p38alpha and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1.J Biol Chem. 2001 May 11;276(19):16491-500. doi: 10.1074/jbc.M010966200. Epub 2001 Jan 30. J Biol Chem. 2001. PMID: 11278799
-
Enzymatic activity and substrate specificity of mitogen-activated protein kinase p38alpha in different phosphorylation states.J Biol Chem. 2008 Sep 26;283(39):26591-601. doi: 10.1074/jbc.M801703200. Epub 2008 Jul 31. J Biol Chem. 2008. PMID: 18669639 Free PMC article.
-
Discordance between the binding affinity of mitogen-activated protein kinase subfamily members for MAP kinase phosphatase-2 and their ability to activate the phosphatase catalytically.J Biol Chem. 2001 Aug 3;276(31):29440-9. doi: 10.1074/jbc.M103463200. Epub 2001 May 31. J Biol Chem. 2001. PMID: 11387337
-
Dual-specificity MAP kinase phosphatases (MKPs) and cancer.Cancer Metastasis Rev. 2008 Jun;27(2):253-61. doi: 10.1007/s10555-008-9123-1. Cancer Metastasis Rev. 2008. PMID: 18330678 Review.
-
DUSP9, a Dual-Specificity Phosphatase with a Key Role in Cell Biology and Human Diseases.Int J Mol Sci. 2021 Oct 26;22(21):11538. doi: 10.3390/ijms222111538. Int J Mol Sci. 2021. PMID: 34768967 Free PMC article. Review.
Cited by
-
mTORC2 modulates feedback regulation of p38 MAPK activity via DUSP10/MKP5 to confer differential responses to PP242 in glioblastoma.Genes Cancer. 2014 Nov;5(11-12):393-406. doi: 10.18632/genesandcancer.41. Genes Cancer. 2014. PMID: 25568665 Free PMC article.
-
Dual-Specificity Phosphatase Regulation in Neurons and Glial Cells.Int J Mol Sci. 2019 Apr 23;20(8):1999. doi: 10.3390/ijms20081999. Int J Mol Sci. 2019. PMID: 31018603 Free PMC article. Review.
-
Dual-specificity Phosphatase 9 protects against Cardiac Hypertrophy by targeting ASK1.Int J Biol Sci. 2021 May 27;17(9):2193-2204. doi: 10.7150/ijbs.57130. eCollection 2021. Int J Biol Sci. 2021. PMID: 34239349 Free PMC article.
-
A high-throughput assay for phosphoprotein-specific phosphatase activity in cellular extracts.Mol Cell Proteomics. 2013 Mar;12(3):797-806. doi: 10.1074/mcp.O112.024059. Epub 2012 Dec 11. Mol Cell Proteomics. 2013. PMID: 23233447 Free PMC article.
-
Dual-specificity MAP kinase phosphatases (MKPs): shaping the outcome of MAP kinase signalling.FEBS J. 2013 Jan;280(2):489-504. doi: 10.1111/j.1742-4658.2012.08716.x. Epub 2012 Aug 28. FEBS J. 2013. PMID: 22812510 Free PMC article. Review.
References
-
- Owens D. M., Keyse S. M. (2007) Oncogene 26, 3203–3213 - PubMed
-
- Camps M., Nichols A., Arkinstall S. (2000) FASEB J. 14, 6–16 - PubMed
-
- Dickinson R. J., Keyse S. M. (2006) J. Cell Sci. 119, 4607–4615 - PubMed
-
- Muda M., Theodosiou A., Rodrigues N., Boschert U., Camps M., Gillieron C., Davies K., Ashworth A., Arkinstall S. (1996) J. Biol. Chem. 271, 27205–27208 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

